Identification of a key molecule linked to side effects of anti-cancer drugs and resistance to treatment
Research Press Release | December 13, 2013
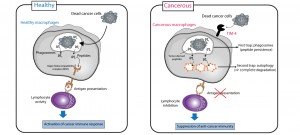
Macrophages in cancer tissue promote the over-degradation of immunoactive substances (such as peptides) derived from cancer cells, via TIM-4.
|
Press Release |
||
|---|---|---|
| Key Points | ・The TIM-4 protein (T-cell immunoglobulin-and mucin domain-containing molecule-4), which is expressed in macrophages, contributes to immunosuppression, a side effect of anti-cancer drugs.
・TIM-4 has been identified as a critical factor in suppressing the therapeutic efficacy of anti-cancer drugs by over-degrading cancer immunoactive substances (peptides). ・TIM-4 inhibitors increase cancer-specific immunogenicity and improve the therapeutic efficacy of anti-cancer drugs. |
|
| Overview |
Immunosuppression has been known to be one of the main side effects of anti-cancer drugs, but it remains unclear about the detailed mechanism of its appearance and its effect on the therapeutic efficacy of anti-cancer drugs. This study revealed a new mechanism for the simultaneous development of immunosuppression and resistance to cancer drugs, whereby anti-cancer-drugs inhibit the process of immunoactive peptide synthesis, which is an important function of macrophages, the most important type of immunocompetent cell in cancer tissue. In practical terms, the expression of a protein called TIM-4 has now been identified in macrophages, but only those in cancer tissue. TIM-4 was found to over-degrade immunoactive peptides synthesized by cancer cells, preventing cancer-specific lymphocyte activity and causing immunosuppression. Immune response activation by TIM-4 inhibitors both alleviated side effects and improved the therapeutic efficacy of anti-cancer drugs. These results are highly significant as they form a link between the side effects of anti-cancer drugs and treatment resistance, demonstrating for the first time worldwide both the expression of cancer-specific immune substances in cancer tissue and their detailed mechanism of action. In addition, these results are ground-breaking as they indicate the possibility that regulating proteins derived from cancer tissue-specific macrophages may lead to the development of new types of cancer treatments with few side effects and high therapeutic efficacy. |
|
| Inquiries |
Masahisa Jinushi, Associate Professor, Institute for Genetic Medicine, Hokkaido University TEL: +81-11-706-6073 FAX: +81-11-706-6071 E-mail: jinuchi@igm.hokudai.ac.jp Research Center for Infection-associated Cancer |
|
|
Japanese Link |
抗がん剤による副作用と治療耐性をつなぐ「鍵分子」を同定 | |
| Publications | Immunity (2013.12.5) | |
