Rapid Synthesis of Zero Waste Chiral Oxindoles Successfully Achieved
Research Press Release | March 18, 2014
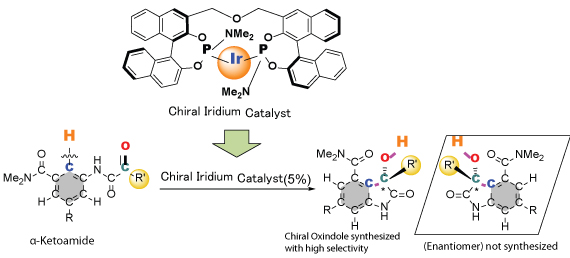
Asymmetric intramolecular direct hydroarylation of α-ketoamides
| Press Release | ||
|---|---|---|
| Key Points |
・A method to synthesize chiral oxindoles by rearrangement without emitting waste was developed. ・Using an original catalyst, selective fragmentation of carbon-hydrogen bond and asymmetric carbon-carbon bond formation were successfully performed. ・The economic and rapid synthesis of chiral compounds, which are the raw material for medicine, is expected. |
|
| Overview | A method of synthesizing chiral oxindoles without emitting waste has been successfully developed. An original catalyst was used to carry out selective fragmentation of specific carbon-hydrogen bond of α-keto amide molecule and formation of new carbon-carbon bond, resulting in the successful synthesis of almost pure chiral oxindoles. Chiral oxindoles are used as raw material for medicine and drugs, and in the future, rapid synthesis of candidate medicine is anticipated. | |
| Inquiries |
Yasunori Yamamoto, Specially Appointed Associate Professor, Frontier Chemistry Center (Jpn), Faculty of Engineering TEL & FAX:+81-11-706-6560 E-mail: yasuyama@eng.hokudai.ac.jp |
|
|
Japanese Link |
廃棄物ゼロでキラルオキシインドール類の迅速合成に成功 | |
| Publications |
Angewandte Chemie International Edition (2014.2.20) |
|
