Fine chemicals made from discarded crab shells by chemical and mechanical force
Research Press Release | November 24, 2015
| Press Release | ||
|---|---|---|
| Key Points |
・N-Acetylglucosamine and its derivative are synthesized from chitin contained in crab shell. ・Combination of small amount of H2SO4 and mechanical force achieves selective conversion of chitin. ・We hope that chitin will be a renewable resource for production of chemicals. |
|
|
Outline |
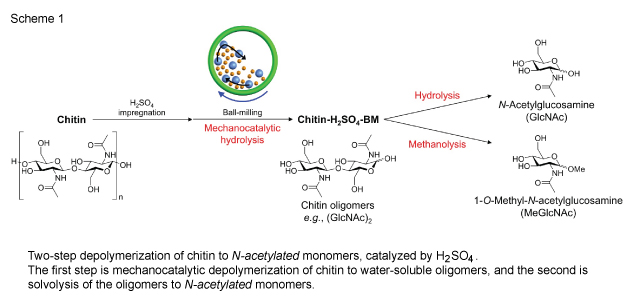
The shell of crustaceans such as crab and prawn is primarily made up of chitin. It is estimated that about 10 billion tons of chitin is produced annually. Chemically, chitin is a polymer of an aminosugar 1, N-acetylglucosamines, which is a feedstock for production of pharmaceutical compounds and functional polymers. However, the conversion of chitin to the monomer currently requires large amount of hydrochloric acid or a long-time reaction with enzymes. These issues limit the use of N-acetylglucosamine to health food purpose. Herein, the group of Prof. Fukuoka and Dr. Kobayashi achieved high yield production of N-acetylglucosamine and its derivative by combining a small amount of sulfuric acid and mechanical force (Figure 1). This process reduces the use of acid by 99.8%. This study was supported by Grant-in-Aid for Young Scientists (A) No. 26709060 and that for research fellows. |
|
|
Background/ Research Methods |
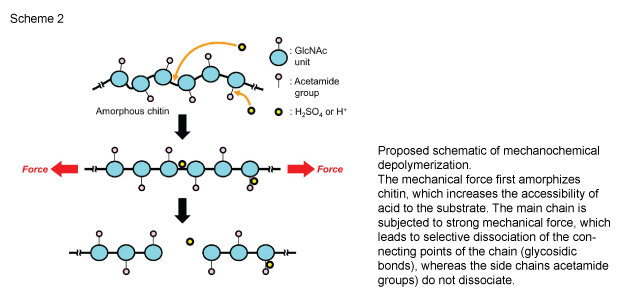
(Background) Biomass has attracted great attention as a renewable resource because it can serve as an alternative to fossil fuel derived chemicals. Chitin is the second most abundant biomass on our planet next to cellulose. It is found in various organisms, especially in exoskeleton of crustaceans like crabs and prawns. Chitin contains nitrogen atoms as it is a polymer of an aminosugar, N-acetylglucosamine. This property enables the use of chitin for a wide range of value-added chemicals such as pharmaceuticals and functional polymers. However, the production of N-acetyl glucosamine and its derivatives requires large amount of hydrochloric acid or a long-time reaction with enzymes due to the recalcitrance of chitin. Hence, the current processes are cost-consuming, and most part of chitin is discarded except for small-scale purposes, e.g., health food. Therefore, we need a new process for the efficient utilization of chitin. (Research Method) Chitin contains glycosidic bonds connecting N-acetylglucosamine units and amide bonds hanging from the units in the molecule (Figure 2). If we can selectively dissociate glycosidic bonds, N-acetylglucosamine is obtained. However, it is known that both bonds are cut when a small amount of acid is used in conventional method. Here, we developed the method to use mechanical tension on chitin molecule in the presence of small amount of acid. This combination selectively cleaves glycosidic bonds due to high tensile stress on the chemical bonds. |
|
|
Research Results/ Anticipated Outcomes |
(Results) Chitin was impregnated with a small amount of sulfuric acid and mechanical force was applied by planetary ball mill 2. This treatment gave oligosaccharides consisting of several N-acetylglucosamine units. The intermediate was converted to N-acetylglucosamine in hot water and 1-O-methyl-N-acetylglucosamine in hot methanol in high yields (Figure 1). This process decreased the use of acid by 99.8%, compared with conventional processes relying on only acid. (Anticipated Outcomes) N-acetylglucosamine and 1-O-methyl-N-acetylglucosamine are precursors to anticancer and anitivirus agents, and other potential applications such as functional polymers also have been shown. Our system provides facile synthesis of the aminosugars using only a small amount of acid, which is going to enhance the use of chitin. Moreover, crabs and shrimps are typical staples in Hokkaido, and we hope that the use of discarded part (shell) will further invigorate regional economies. |
|
|
Terms |
1 Aminosugar: Typical sugars contain carbon, hydrogen, and oxygen atoms (e.g., glucose, sucrose). Aminosugar is a sugar that includes nitrogen atoms (more accurately amino group) in addition to the three elements. N-acetylglucosamine is the most famous aminosugar. 2 Planetary ball mill: Sulfuric acid-impregnated chitin and alumina balls are charged in a alumina pot. The pot is rotated in high speed. This spin includes both rotation and revolution like movement of planets. This movement causes impact between balls and between balls and wall of the pot. As a result, chitin gets sandwiched between the two colliding bodies and is exposed to tensile force, which results in breaking of the glycoside bond. |
|
| Inquiries |
Atsushi FUKUOKA, Professor, Fukuoka Lab, Institute for Catalysis, Hokkaido University FAX: +81-11-706-9139 E-mail: fukuoka[at]cat.hokudai.ac.jp |
|
|
Japanese Link |
カニの甲羅を触媒と機械的な力で機能化学品に変える (11.17.2015) | |
| Publications | Catalytic Depolymerization of Chitin with Retention of N-Acetyl Group, ChemSusChem (11.5.2015) | |