The Water Layer that Wets an Ice Surface does not Flow as Easily as Ordinary Water
Research Press Release | January 21, 2016
| Press Release | ||
|---|---|---|
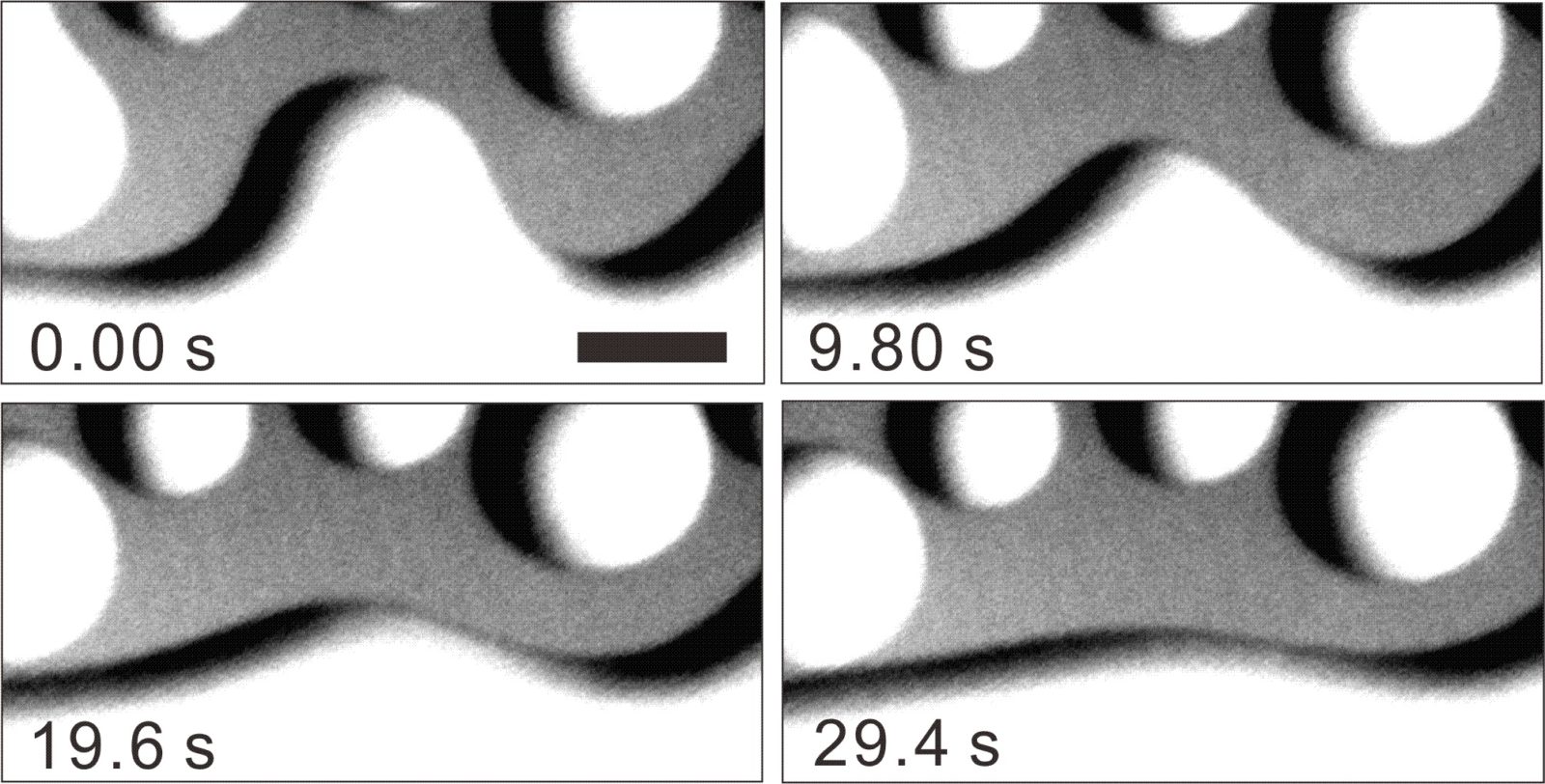
| Key Points | ・By tracing morphological changes of quasiliquid layers (QLLs)1 with advanced optical microscope in a noninvasive way , we succeeded in quantitatively evaluating the physical properties2 of QLLs, which had been difficult to measure in the past.
・We clarified that the properties of QLLs are clearly different from those of bulk water3. ・We present a new approach for identifying the mechanism of ice surface melting, which has been a mystery for many years. |
|
|
Outline |
Ice surfaces are wet by a thin water layer even below 0°C. This phenomenon has been known as “surface melting” for a long time, but there are still major questions as to why the ice surface is wet. This research has focused on “quasiliquid layer (QLLs)” water layer that is formed by surface melting. We investigated its physical properties by using an advanced optical microscope. As a result, we found that the ability of QLLs to flow (the surface tension-to-shear viscosity ratio) is reduced to a maximum of 1/200 that of bulk water. In other words, the water layer that wets the ice surface does not flow as easily as bulk water does. It is strongly suggested that this difference is caused by the fact that water molecules distributed locally on ice interface have a unique structure and dynamics that are not seen in bulk water. We expect that our findings will be an important clue toward identifying the mechanism of ice surface melting, which has been a mystery for many years. |
|
|
Anticipated Outcomes |
It is believed that QLLs on an ice crystal is closely related to low-temperature natural phenomena in our daily life, such as snowball making, the lubricity of ice surfaces, frost heaves, morphological changes of snow, and the electrification of thunderclouds. Thus, we hope that an accurate and direct evaluation of the physical properties of QLLs, as obtained in this research, will provide important knowledge for understanding these natural phenomena. Also, our experimental results show that the flow of water near an ice interface is greatly different from that of bulk water, and we expect that the anomalous behavior of water caused by the ice interface is a universal phenomenon that can occur with various other interfaces besides ice. With recent advances in nanotechnologies, research into the fluid flow characteristics of water within so-called “nanospaces” (microfluidics, nanofluidics) is getting attention. The effect of a confining wall, that is, an interface is of crucial, within an extremely small space at the nanoscale level. For example, the flow of water within a biological cell are also being considered as targets of microfluidics and nanofluidics. Therefore, we expect that our research results will extend beyond condensed matter physics and have a large impact across many research areas. |
|
|
Terms |
1. Quasiliquid layers (QLLs): An extremely thin water layer that covers an ice surface, and this formed by surface melting. Its thickness is estimated to be about several tens of nanometers, as measured by using ellipsometry. This water layer is predicted to be water that has a structure closer to that of ice, so it is called a “quasiliquid layer” to distinguish it from normal bulk water3. 2. Physical Properties: General name for properties of substances, such as density, refraction index, specific heat, melting point, and boiling point. In this research we focus on the ratio between the surface tension and the shear viscosity of QLLs. 3. Bulk Water: It is known that in substances (not limited water) whose size is on the scale of nanometers, physical properties can remarkably change depending on size. This is called a finite-size effect. Bulk water means water that is large enough (at least micrometers in size) so that a finite-size effect does not occur. |
|
| Inquiries |
Institute of Low Temperature Science, Hokkaido University Assis. Prof. Ken-ichiro MURATA TEL:+81-11-706-5466 murata[at]lowtem.hokudai.ac.jp |
|
|
Japanese Link |
氷の表面を濡らす水膜は普通の水より流れにくい (12.18.2015) | |
| Publications | In situ Determination of Surface Tension-to-Shear Viscosity Ratio for Quasiliquid Layers on Ice Crystal Surfaces (12.18.2015) | |
