Research Press Release | February 16, 2016
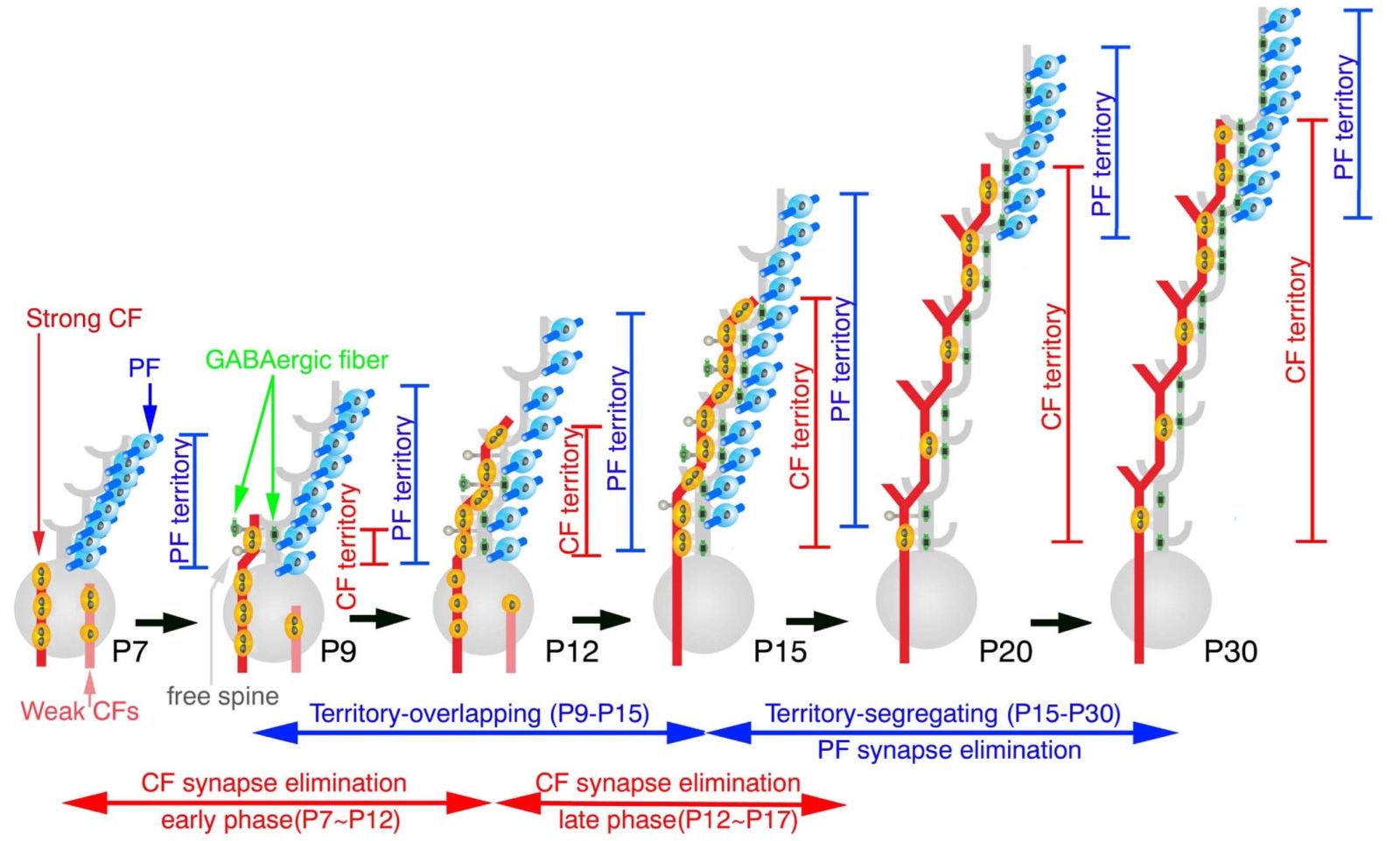
Fig. 1. Developmental changes in innervation territories by parallel fibers and climbing fibers in cerebellar Purkinje cells.
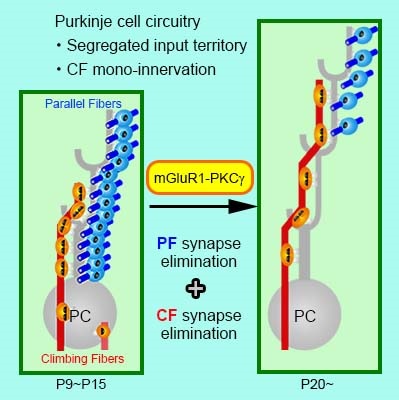
Fig. 2. mGluR1-PKCg signaling pathway promotes synaptic circuit refinement and maturation in cerebellar Purkinje cells.
|
Key Points
|
-
Purkinje cells1 (PCs) in the cerebellum are involved in skilled motor function and motor learning. These functions underlie unique synaptic circuits on PC dendrites comprising innervation of proximal dendrites by single climbing fibers2 (CFs) and of distal dendrites by hundreds of thousands parallel fibers3 (PFs).
-
So far, we have clarified that mono-innervation of PCs by single CFs is established by homologous competition among CFs and heterologous competition between CF and PF, and that these competitions are fueled by molecular mechanisms controlling activity-dependent intracellular Ca2+ rise and input-selective synaptic adhesion.
-
Here we report that CF and PF territories on PC dendrites segregate through PF synapse elimination4 from proximal dendrites, and this segregation is promoted by metabotropic glutamate receptor5 mGluR1 expressed in PCs.
|
|
Outline
|
Immature synaptic circuits are refined into mature functional ones by strengthening of active or used synapses and elimination of inactive or unused synapses in early postnatal development. In CF innervation of cerebellar PCs, this refinement is achieved through CF synapse elimination until mono-innervation by single CF is established. However, whether and how innervation by PFs, another input to PCs, is refined remains unknown. In the present study, we clarify that PF synapses are initially formed all over the dendritic tree of PCs until postnatal day 15 in mice, and that innervation territories by PFs and CFs segregates by PF synapse elimination from proximal PC dendrites during postnatal day 15-20. We further demonstrate that metabotropic glutamate receptor mGluR1 plays an essential role in the PF synapse elimination.
Our findings were published on February 8, 2016 in on-line Early Edition of Proceedings of the National Academy of Sciences of the United States of America. This study was supported by Grants-in-Aid for Scientific Research (24220007) and the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from AMED.
|
| Terms |
- Purkinje cell (PC)
The PC is the principal neuron in the cerebellum, and involved in motor coordination and learning. This neuron receives neural inputs concerning motor planning from the cerebrum and sensory information from the muscle, tendon, and joint, and enables skillful movement through minimizing the gap between them.
- Climbing fiber (CF)
The CF is projection fibers from the inferior olive in the medulla. This fiber conveys ‘error’ signals relevant to the gap between motor plan and its outcome to proximal dendrites of PCs. The climbing fiber elicits strong depolarization in the innervating PCs, triggering massive Ca2+ influx into PC dendrites by activating voltage-dependent Ca2+ channels.
- Parallel fiber (PF)
The PF conveys motor information from the cerebral cortex and sensory information from the muscle, tendon, and joint via the spinal cord and brainstem, both to distal dendrites of PCs. When PF synapses are activated in conjunction with CF depolarization, long-term depression, a form of synaptic plasticity underlying motor learning, is induced at the PF synapses.
- Synapse elimination (pruning)
Following a surge in synapse numbers and densities in neonates, active or used synapses are strengthened, while inactive or unused synapses are eliminated. This process is called the synapse pruning, through which synaptic circuits are dynamically refined during early postnatal file called the critical or sensitive period. Synapse pruning drives the acquisition of mother tongue and robust improvement in sports skills and musical instrumental performance in childhood.
- Glutamate receptor
There are several subtypes in ionotropic glutamate receptors, of which AMPA-type and kainate-type receptors mediate fast excitatory synaptic transmission, and NMDA-type receptor is involved in synaptic plasticity. Moreover, the delta-type ionotropic glutamate receptor, which loses ion channel activity and glutamate binding, mediates input-selective synaptic adhesion. There are also several subtypes in metabotropic glutamate receptors, of which mGluR1 is richly expressed in PCs and involved in Ca2+ release from intracellular Ca2+ store and protein kinase activation.
|
| Inquiries |
Masahiko WATANABE, Professor
Department of Anatomy and Embryology, Graduate School of Medicine
watamasa[at]med.hokudai.ac.jp
|
|
Japanese Link
|
代謝型グルタミン酸受容体 mGluR1 はシナプス刈込みを駆動して 小脳神経回路を成熟させる (02.09.2016) |
|
Publications
|
Territories of heterologous inputs onto Purkinje cell dendrites are segregated by mGluR1-dependent parallel fiber synapse elimination, Proceedings of the National Academy of Sciences of the United States of America (02.09.2016) |


