|
Key Points
|
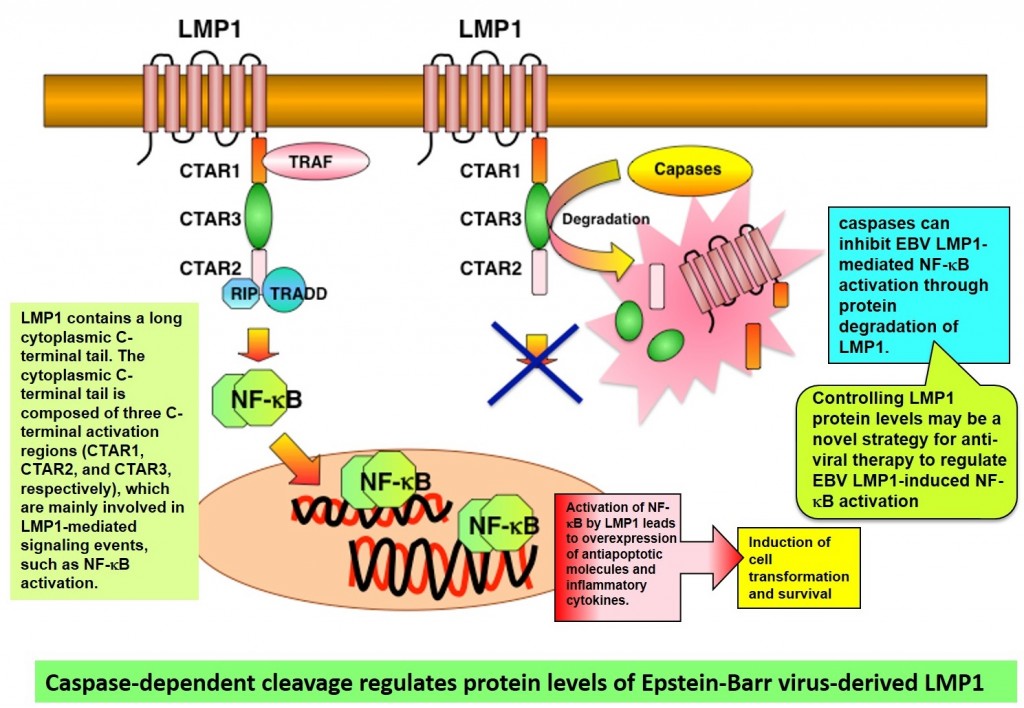
- Expression of a cancer virus product, latent membrane protein 1 (LMP1), in cells leads to activation of caspase1, a type of protease, resulting in LMP1 degradation.
- When expression of caspases that degrade the cancer virus product LMP1 is suppressed, activation of the transcription factor NF-κB2 and production of inflammatory cytokines are enhanced.
- There are hopes for development of novel antiviral agents that target the mechanism that regulates protein levels of LMP1.
|
|
Background
|
Epstein-Barr virus, which is known to be a cancer virus, is a herpesvirus that infects most adults. It is known to be associated with nasopharyngeal carcinoma, Hodgkin lymphoma, and stomach cancer, and virus products expressed in association with Epstein-Barr viral infection are linked to cancer development.
We have identified a novel control mechanism for degradation of latent membrane protein 1 (LMP1), which is known to be an Epstein-Barr virus product with a particularly close connection with cancer development.
In cells that have been treated with caspase inhibitor, or with which gene knockdown3 has been performed, LMP1 degradation is suppressed, and NF-κB signals, which support inflammation, are found to be enhanced. We hope to develop novel antiviral agents that target LMP1 protein control.
|
| Terms |
- Caspases: Caspases are a family of factors that induce apoptosis, or cell death. They are cysteine-aspartic proteases, with cysteine central to their activity, at the C-terminal ends of the aspartate residues (D) of matrix proteins. Fourteen types of caspase are known to occur in mammals, and among these it is primarily caspase-8 and caspase-9 that act in the initial phase of apoptosis induction, with caspase-3 and caspase-7 being active primarily in the apoptosis achievement phase.
- Transcription factor NF-κB: Nuclear factor κB (NF-κB) was initially identified as a transcription factor that binds to the κ-light-chain-enhancer of immunoglobulins expressed selectively in B cells, but it has since been shown to be expressed by almost all cells, including the cells of invertebrates, such as urchins and fruit flies, as well as higher animals. In mammals in particular, five molecules that belong to the NF-κB family, also called as the Rel family, are known. Members of the NF-κB family are involved in various biological phenomena, such as the immune response and cell viability, through induction of expression of inflammatory cytokines, antiapoptotic factors, etc. In addition, NF-κB activation is dysregulated in various diseases, including inflammatory and autoimmune diseases, and cancer, and attention is therefore being given to NF-κB as target molecules for treatment of various diseases.
- Gene knockdown: This refers to gene knockdown using small interfering RNA (siRNA). SiRNA is low-molecular-weight, double-stranded RNA containing 21 to 23 base pairs that is involved in the phenomenon of RNA interference (RNAi). In RNAi, breakdown of mRNA results in sequence-specific inhibition of gene expression, and it can therefore reduce the transcription levels of specific genes, and markedly weaken gene function. At the present time, RNAi using siRNA as a gene knockdown method is applied to fundamental research in the fields of biology and pharmacology, and it is hoped that clinical applications will also be found in future.
|
| Inquiries |
Professor Tadashi MATSUDA
Department of Immunology, Graduate School of Pharmaceutical Sciences, Hokkaido University
tmatsuda[at]pharm.hokudai.ac.jp
|
|
Japanese Link
|
がんウイルス産物の新たな分解制御機構の同定 (03.07.2016) |
|
Publications
|
Caspase-dependent cleavage regulates protein levels of Epstein–Barr virus-derived latent membrane protein 1, FEBS letters. (02.27.2016)
|