Carbon-11 labeled Methionine PET study for diagnosis of brain tumor recurrence will start based on Advanced Medical Care B Program
Research Press Release | February 24, 2015
| Press Release | ||
|---|---|---|
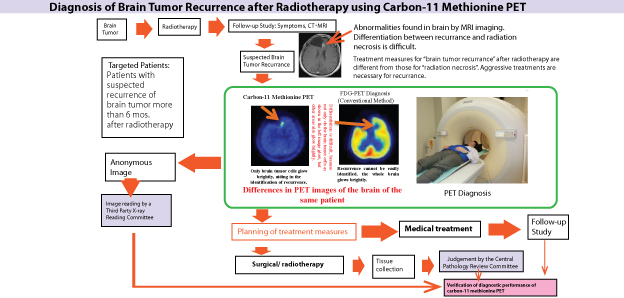
| Key Points | ●Carbon-11 labeled Methionine*1 PET study (Met-PET study) for diagnosis of brain tumor recurrence after radiation therapy was approved in advanced medical care B program*2 The study will begin at Hokkaido University Hospital.
● The Met-PET study is expected to be more useful than conventional imaging diagnostic methods in the diagnosis of brain tumor growth, brain tissue necrosis caused by radiotherapy, and recurrence of brain tumors. ●The differential diagnosis between tumor recurrence and radiation necrosis with MRI is sometimes difficult because in both cases, MRI pattern is characterized by an increased gadolinium contrast-enhancement. With Met-PET, the differential diagnosis will become more correctly and the patients will be treated appropriately. |
|
| Overview |
Carbon-11 labeled Methionine PET study (Met-PET study) for diagnosis of brain tumor recurrence after radiation therapy was approved in advanced medical care B program. The clinical study will begin at Hokkaido University Hospital in January, 2015. The Met-PET study has been led by Professor Nagara Tamaki from the Department of Nuclear Medicine, Hokkaido University Graduate School of Medicine, and others. This method is expected to be more useful than conventional imaging methods in the deliniation of brain tumor extent, and differential diagnosis between brain tumor recurrence and radiation necrosis. This clinical study aims to verify the superiority and safety of the Met-PET study over conventional methods, and to establish the diagnostic method using the Met-PET study to determine brain tumor recurrence. Explanation of Terms: *1) Carbon-11-labeled methionine: Methionine is an essential amino acid in the formation of proteins. Carbon-11-labeled methionine becomes radioactivated methionine by being labeled with radioactive carbon-11 and has the unique property of being able to be absorbed into tumor cells, to clearly identify tumors. *2) Advanced Medical Care B: “Advanced Medical Care” healthcare system has been established by the Ministry of Health, Labour and Welfare in Japan, which allows co-payment for medical treatment deemed as advanced medical treatment etc. used in R&D at a medical institution, by the national health insurance and the patient (mixed medical treatment). “Advanced Medical Care B” is equivalent to former high-level medical care. Approval, certification, and application as specified in the Pharmaceutical Affairs Act are not required, but confirmation of safety and efficacy is a prerequisite for this category. |
|
| Inquiries |
Tohru Shiga, Associate Prof., Department of Nuclear Medicine (page is in Japanese), Hokkaido University Hospital TEL: +81-11-706-5151 FAX: +81-11-706-7155 E-mail:kakui-s@med.hokudai.ac.jp |
|
|
Japanese Link |
||
