Uncovering the Mechanism Behind Stressed Naïve T-cells In vivo
Research Press Release | July 02, 2015
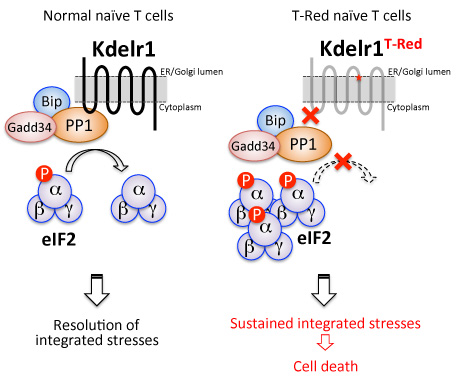
In normal naïve T-cells, association between Kdelr1 and PP1 reduces the phosphorylation of eIF2α, effectively resolving integrated stress of naïve T-cells followed by their survival. In T-Red naïve T-cells with a point mutation in the Kdelr1 gene, Kdelr1 cannot associate with PP1, and PP1 cannot dephosphorylate eIF2α. Therefore, in T-Red naïve T-cells, phosphorylation of eIF2α is excessively maintained, and cell death is induced by unnecessary continuation of integrated stress responses.
| Press Release | ||
|---|---|---|
| Key Points | • We found an ENU-mutant mouse line (T-Red) with reduced numbers of naïve T-cells. • A point mutation1 in the KDEL receptor1 (Kdelr1) gene, which leads its loss-of-function, was identified as the cause. • The association of Kdelr1 with a phosphatase, PP1 reduces stress responses in naïve T-cells through dephosphorylation of eIF2α. • This discovery is expected to develop methods to artificially control naïve T-cell functions, which are critical for therapies against autoimmune diseases, infectious agents, and cancers. |
|
| Overview |
Our immune system is critical in suppressing infections and cancers, but its over-activation can lead to allergies and autoimmune diseases. This research focused on T-cells, or white blood cells. The immune system is evolutionally developed as a defense mechanism against pathogens. The number of T-cells in the body is constant, and they react to foreign antigens on pathogens efficiently. We established mutant mouse lines with abnormal numbers of T-cells by using the mutagen ENU2, in order to gain new information regarding the mechanism involved in the adjustment in the number of T-cells in vivo. We identified mutant mice (T-Red mice) with reduced numbers of naïve T-cells, and found that this is caused by a mutation in the Kdelr1 gene, which leads its loss-of-function. In T-Red mice, excess naïve T-cell death was observed due to accumulated stress responses. Mechanistic analysis shows that KDELR1 directly regulates protein phosphatase 1 (PP1), a key phosphatase for stress responses in naïve T-cells. T-Red KDELR1 does not associate with PP1, resulting in reduced phosphatase activity against eIF2α and subsequent expression of stress responsive genes including the proapoptotic factor BIM. These results demonstrate that KDELR1 regulates naïve T-cell homeostasis by controlling ISR3. This knowledge is expected to develop methods to artificially control naïve T-cell functions, which are critical for therapies against autoimmune diseases, infectious agents, and cancers. 1 Point mutation |
|
| Inquiries |
Masaaki MURAKAMI, Professor, Molecular Neuroimmunology, Institute for Genetic Medicine, Hokkaido University TEL: +81-11-706-5120 FAX: +81-11-706-7542 E-mail: murakami@igm.hokudai.ac.jp |
|
|
Japanese Link |
T リンパ球がストレスを解消する機構を解明 | |
| Publications | KDEL receptor 1 regulates T-cell homeostasis via PP1 that is a key phosphatase for ISR, Nature Communications (2015.6.17) | |