A “key” to metastasis formation
Research Press Release | July 13, 2016
Cells lining the walls of tumour blood vessels hold a “key” that allows tumour cells to break into the blood stream and form metastases, say researchers at Hokkaido University.
Researchers at Hokkaido University in Japan have demonstrated that a molecule, called biglycan, plays an intrinsic role in attracting tumour cells toward the inner wall of tumour blood vessels.
Biglycan is a normal component of the supportive matrix outside cells and appears to modulate the biological activities of a number of growth factors. It is also released by immune cells in inflamed tissues.
|
|
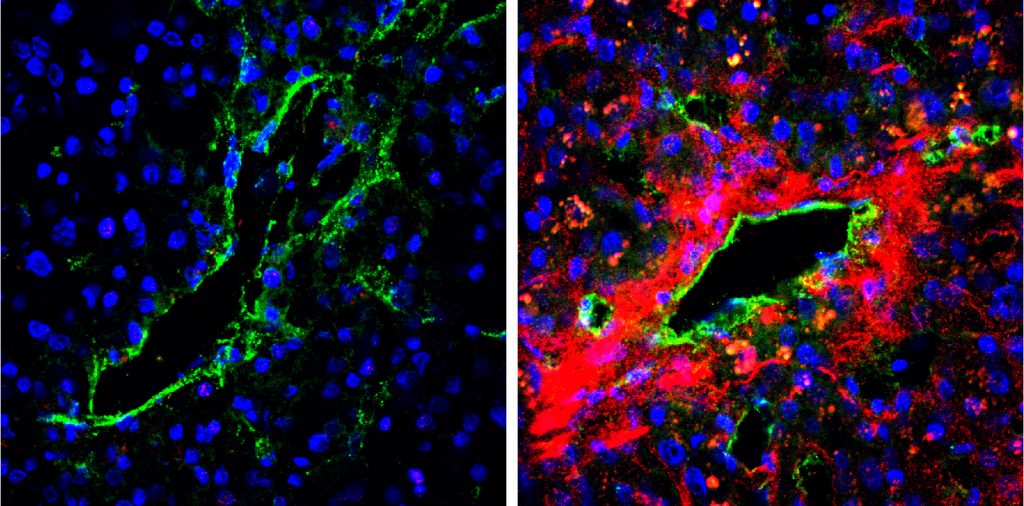
| The secretion of biglycan in tumour blood vessels of cancer patients. A strong biglycan signal (red) was detected in the tumour blood vessel area of a metastatic case (right) but was barely detected in the tumour tissues of a non-metastatic case (left). (Maishi N. et. al., Scientific Reports. June 13, 2016) |
The team conducted investigations by grafting low-metastatic (LM) and high-metastatic (HM) melanoma tumour cells under the skin of mice.
They found the “endothelial” cells forming the inner lining of blood vessels in the HM tumours secrete increased levels of biglycan, which then binds to specific receptors on the tumour cells, attracting them to the endothelium. When they neutralized the biglycan receptors with antibodies, tumour cell migration toward the endothelium was inhibited.
They then asked why biglycan expression was increased in the endothelial cells, and found that the promoter region of the gene was de-methylated, meaning the gene was switched on. They speculate that the tumour cells created a micro-environment that could induce abnormal gene expression in the neighbouring tissue.
The team analysed a large database that contains gene expression data in cancer patients. They found that high biglycan expression was associated with poor prognosis in patients with breast, lung and colorectal cancer. Further investigation on a specific metastatic cancer case showed high levels of biglycan in the blood of patients with metastatic cancer, while it was barely detected in another non-metastatic case.
The research provides clear evidence that biglycan plays an important role in determining tumour malignancy. Blood vessel endothelial cells act as “gatekeepers”, the researchers write in their study published in the journal Scientific Reports. In highly metastatic tumours, these cells give tumour cells the “key” molecule, biglycan, which allows them to break through the gate and proceed into the blood stream, resulting in metastases.
“The present observations, together with unravelling certain remaining issues, may contribute to establishing accurate diagnostics or potent anti-metastatic strategies that target the communications between tumour cells and endothelial cells,” the researchers conclude.
|
|
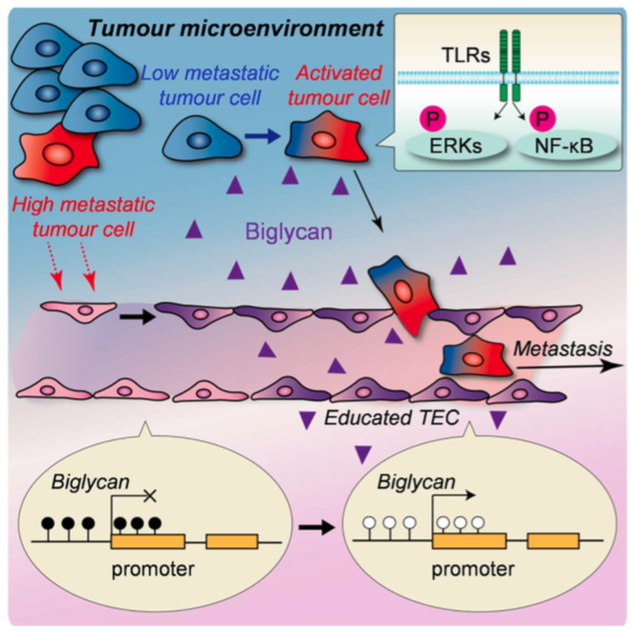
| Schematic view of biglycan’s role in metastasis. Tumour endothelial cells (TEC) affected by the tumour microenvironment express biglycan, which allows tumour cells to break through the gate and proceed into bloodstream. (Maishi N. et. al., Scientific Reports. June 13, 2016) |
Original article:
Maishi N. et. al., Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan. Scientific Reports. June 13, 2016. DOI: 10.1038/srep28039
Contacts:
Associate Professor Kyoko HIDA
Laboratory for Vascular Biology
Institute for Genetic Medicine
Hokkaido University
Khida[at]igm.hokudai.ac.jp
Naoki Namba (Media Officer)
Global Relations Office
Office of International Affairs
Hokkaido University
Email: pr[at]oia.hokudai.ac.jp
Tel: +81-11-706-8034