A novel method to remove a pathological agent of Alzheimer’s Disease : administration of cell-derived nanoparticles reduces accumulation of amyloid beta in the animal brain
Research Press Release | August 20, 2014
-
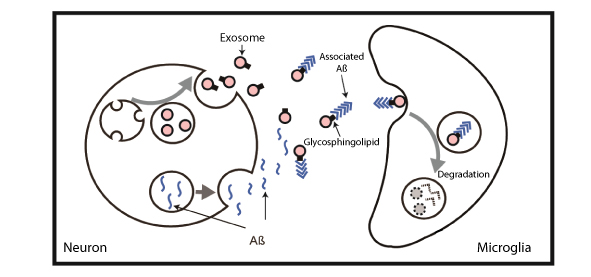 Clearance of Aß from the brain by the administration of exosomes. (pic 1) Both exosomes and Aß are produced in neurons, and secreted outside the cell. Exosomes can capture Aß by glycosphingolipids on the membrane surface, and transport it to microglia where it is broken down. When exsomes were continuously administered to the brain of an APP transgenic mouse, a reduction in the accumulation of Aß in the hippocampus was observed.
Clearance of Aß from the brain by the administration of exosomes. (pic 1) Both exosomes and Aß are produced in neurons, and secreted outside the cell. Exosomes can capture Aß by glycosphingolipids on the membrane surface, and transport it to microglia where it is broken down. When exsomes were continuously administered to the brain of an APP transgenic mouse, a reduction in the accumulation of Aß in the hippocampus was observed. -
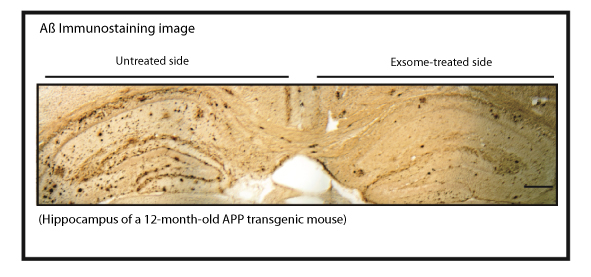 Clearance of Aß from the brain by the administration of exosomes. (pic 2)
Clearance of Aß from the brain by the administration of exosomes. (pic 2)
| Press Release | ||
|---|---|---|
| Key Points | By administering exosomes1, cultivated cell-derived nanoparticles, we successfully reduced the concentrations of amyloid beta-peptide (Aß) 2 and suppressed the accumulation of amyloid plaque3.
・Sugar chains of glycosphingolipids4 are involved in the binding of Aß to exosomes. ・This new knowledge raises the possibility that exosomes are involved in the pathogenesis of Alzheimer’s disease, and is expected to contribute to the development of new strategies for treating the disease. |
|
| Overview | One cause of Alzheimer’s disease (AD) is the accumulation of the amyloid beta (Aß) peptide in the brains. Our research team previously identified that Aß binds to cell-derived minute particles (with diameters of 50-100nm) called exosomes, and that this exosome-bound Aß can be transported into microglia5 and broken down in them, by using an experimental system with cultured cells.
This study considered whether the Aß removal function of exsomes can be exhibited in living tissue, using a mouse model of AD. In this study, exsomes collected from the cultured fluid of neuronal cultured cells were administered into mouse brains. As a result, we confirmed that brain Aß bound to the administered exosomes, and were ingested by microglia. Aß concentration, synaptic disorder, and amyloid accumulation were reduced by the continuous administration of exosomes for two weeks by osmotic minipump. Moreover, we determined that glycosphingolipids, a type of membrane glycolipids, were more abundantly contained in exosomes than the derived cells, and that Aß was associated with exosomes through this glycolipid sugar chains. The discovery in this study of exosome function for Aß removal in the brain is expected to lead to new methodologies for the treatment and prevention of AD. This study is executed as a part of “The Matching Program for Innovations in Future Drug Discovery and Medical Care” (Hokkaido University), Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program, Ministry of Education, Culture, Sports, Science and Technology. |
|
| Terms |
1 Exosome: A membrane vesicle secreted from various cells. Exosomes bind to specific molecules, and act as intercellular carriers to deliver bioactive molecules. 2 Amyloid beta-peptide (Aß): Peptides consisting of approximately 40 amino acids which have been cut off and produced from the amyloid beta precursor protein. Excessive accumulation of this peptide is considered to be a trigger for the onset of AD. 3 Amyloid plaque: A deposition of aggregated forms of Aß in the brain. In humans, this is called senile plaque, a main pathology of AD. 4 Glycosphingolipid: A subtype of complex glycolipids containing ceramide and covalently-linked sugar chains. 5 Microglia: A type of glial cells existing in the central nervous system and which shows macrophage-like phagocytic action. |
|
| Inquiries |
Kohei Yuyama, Specially Appointed Assistant Professor, Faculty of Advanced Life Science, Hokkaido University TEL & FAX: +81-11-706-9047 E-mail: kyuyama@pharm.hokudai.ac.jp Yasuyuki Igarashi, Specially Appointed Professor, Faculty of Advanced Life Science, Hokkaido University TEL & FAX: +81-11-706-9001 E-mail: yigarash@pharm.hokudai.ac.jp |
|
|
Japanese Link |
||
| Publications |
The Journal of Biological Chemistry (2014.7.18) |
|
