Caution required for handling genome editing technology
Research Press Release | April 22, 2014
| Research News | |
|---|---|
| Authors |
Motoko Araki (1), Kumie Nojima (2), and Tetsuya Ishii (1) |
|
Key Points |
Genome-editing technology, although a robust tool for genetic engineering, is creating indistinct regulatory boundaries between naturally occurring and modified organisms. However, researchers must act with caution in research and development to avoid misleading society. |
|
Back ground |
Precise genetic engineering can be achieved in higher organisms through genome editing with nucleases such as zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the clustered regularly interspaced short palindromic repeat (CRISPR)/Cas system. Although genome editing has received significant attentionowing to its potential applications in plant and/or animal breeding, it has also raised regulatory issues. The artificial nucleases may generate novel organisms that are extremely similar or identical to naturally occurring organisms. Currently, some countries have attempted to establish regulations for handling ZFNs and TALENs, but not yet the CRISPR/Cas system. Caution is needed because inappropriate use of genome editing may cause societal problems and loss of opportunities for agricultural and environmental applications. Here we briefly review regulatory responses, scrutinize societal implications, and propose a future direction for the biotechnology of genome editing. |
|
Research in Detail |
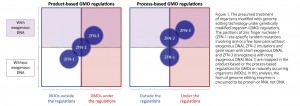
ZFN-1 and ZFN-2 seem to blur the current boundaries of product- and process-based regulations (Figure 1). However, on closer examination, the positions of ZFN-1 and ZFN-2 differ significantly in the product-based versus the process-based regulations. ZFN-1 is outside the scope of product-based regulations but partly within the scope of process-based regulations. This implies that the regulatory position of ZFN-1 depends on whether a country adopts product-based or process- based regulations. By contrast, ZFN-2 has both regulated and unregulated positions, although the existence or use of a short repair template varies its classification by different countries (Figure 1). The current regulatory landscape suggests some directions. By definition, the use of ZFN-3 is regarded as a conventional transgenesis and/or cisgenesis. In the product- based regulations, an efficient assessment method should be required to verify that a product generated using ZFN-1 is outside the regulatory scope. Further scientific and regulatory efforts are needed to minimize the frequency of case-by-case responses to ZFN-1 use under process-based regulations and ZFN-2 under both types of regulation. In both regulatory systems, it is more important to confirm the actual mutations caused by genome editing and whether the mutations cause a functional change that can affect human health or the environment. To explain it differently, the emergence of genome editing technology may provide an important opportunity to form a new global consensus for future regulations in the field of genetic engineering.
Societal implications Although the current regulations are out of step with progress in the field, the efficiency and effectiveness of genome editing in higher organisms does not authorize researchers to advance the application of this technology without caution. The careless use of genome editing would raise social issues and/or repercussions in agricultural and environmental applications. In conventional genetic engineering, the detection of exogenous DNA facilitates the characterization of the resultant organisms. Conversely, some organisms modified with genome editing seem to be almost identical to naturally occurring organisms, implying difficulty in genetically characterizing these organisms. However, such organisms require scientific scrutiny prior to being released into the market and/or into the environment. |
| Conclusions |
Although genome editing demonstrates efficient and effective genetic engineering, this new biotechnology is creating indistinct boundaries in the existing GMO regulations. Under the present conditions, researchers should act with more caution in research and development using genome editing technology compared to traditional genetic engineering technology in the interest of scientific accountability. Most importantly, international harmony is required on this issue, as we experienced a constructive discussion at the Asilomar Conference in 1975 in which researchers, layers, and physicians successfully drew up voluntary guidelines. In order to harness the potential of genome editing for future science and broad applications, researchers, private enterprises, and regulators should proactively discuss and establish appropriate regulations based on a scientific assessment. |
| Inquiries |
Associate Professor Tetsuya Ishii, Office of Health and Safety Hokkaido University |
|
Japanese Link |
To Japanese Press Release http://www.hokudai.ac.jp/news/140419_pr_general. pdf |
|
Source (Extracts from) |
Trends in Biotechnology (Impact factor: 9.660), 32 (2014) pp. 234-237. |

 (click to enlarge)
(click to enlarge)