Clinical Study on New Injection Drug [123I] IIMU for Imaging Diagnosis of Cancer Begins – First-in-Human Studies to be Conducted
Research Press Release | June 24, 2014
| Press Release | ||
|---|---|---|
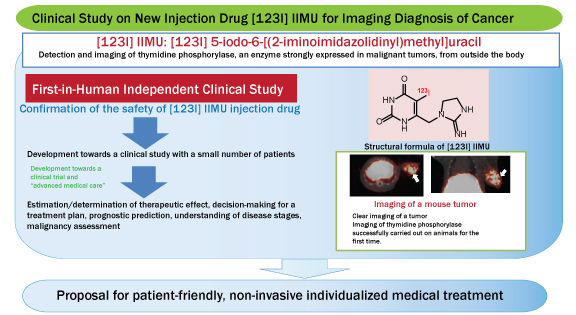
| Key Points | – [123I]IIMU is a compound that readily combines with thymidine phosphorylase, an enzyme strongly expressed in malignant tumors. This “seed” (candidate compound for development) is launched in universities and under development as a new injection drug for imaging diagnosis of cancer.
– A clinical study to verify safety of [123I]IIMU injection drug was approved by the Institutional Review Board of Hokkaido University Hospital for Clinical Research, and First-in-human studies*1 on healthy adults will begin for the first time in the world. – Examinations using [123I]IIMU injection drug are expected to provide useful information that is difficult to obtain from other conventional methods, such estimation/determination of therapeutic effects of anticancer drugs, decision-making for a treatment plan, prognostic prediction, understanding of disease stages, and malignancy assessment. *1: First-in-human study: During new drug development, evaluations are initially carried out on experimental animals through various studies. Efficacy and safety of the test drug are then evaluated on healthy human adult volunteers. This called the “first-in-human study” since the drug is administrated to humans for the first time in the world. |
|
| Overview | Hokkaido University will begin an independent clinical study on [123I] IIMU, a new injection drug for imaging diagnosis of cancer, with Professor Nagara Tamaki of the Graduate School of Medicine as the general supervisor.
The [123I] IIMU injection drug for imaging diagnosis was newly developed by Professor Yuji Kuge and Lecturer Ken-ichi Nishijima of the Central Institute of Isotope Science/Graduate School of Medicine, Hokkaido University, and Professor Kazue Okura of the Graduate School of Pharmaceutical Sciences, Health Sciences University of Hokkaido. Efficacy and safety of the drug were evaluated in preclinical studies.*2 This is the first attempt in the world to administer this injection drug to healthy adults. The purpose of this clinical study is to evaluate the safety of [123I] IIMU injection drug for imaging diagnosis. Examinations using this injection drug for imaging diagnosis are expected to result in the detection and imaging of the activation of thymidine phosphorylase, an enzyme strongly expressed in malignant tumors, from outside the body, as well as provide new information useful in the diagnosis/treatment of cancer patients. *2: Preclinical study: Preliminary study is carried out before the first-in-human study, where the pharmacology (drug actions) and pharmacokinetics (drug behaviors within the body) are evaluated using experimental animals. |
|
| Inquiries |
Yuji Kuge, Professor, Graduate School of Medicine, Hokkaido University TEL: +81-11-706-6087 FAX: +81-11-706-7862 E-mail:IIMU@ric.hokudai.ac.jp |
|
|
Japanese Link |
新しいがんの画像診断用注射薬,[123I]IIMU の臨床研究を開始~First-in-human 試験実施へ~ | |