Deeper insight into how tick spit suppresses cattle immunity
Research Press Release | January 28, 2021
This is a joint press release by Hokkaido University, Japan; Universidade Federal do Rio Grande do Sul, Brazil; and Universidade Federal do Rio de Janeiro, Brazil.
A tick saliva study reveals immune responses that could lead to better protection for cattle.
Scientists from Hokkaido University, Japan and Universidade Federal do Rio Grande do Sul and Universidade Federal do Rio de Janeiro, Brazil, have revealed that substances in tick saliva activates immune response-suppressing proteins in cattle that facilitates the transmission of tick-borne diseases. The finding was published in the journal Scientific Reports and could help in the development of alternative control strategies.
The Asian blue tick, Rhipicephalus microplus, feeds on cattle, causing skin lesions, chronic blood loss and transmission of disease-causing parasites. The costs of preventing and treating disease and loss of some cattle are considerable in many parts of the world.
Some ticks have developed resistance against currently used acaricides, the tick equivalent of insecticides. To develop alternative strategies that can better protect cattle, such as vaccines, scientists need to better understand tick infections at the molecular level. For example, scientists already know that tick saliva suppresses the immune response in cattle, facilitating the transmission of tick-borne parasites, but the exact process has not been fully clarified.
Infectious disease veterinarian, Satoru Konnai, and scientists at Hokkaido University in Japan and colleagues in Brazil investigated what happens to immune cells when they are exposed to tick saliva. The team found that substances in tick saliva, likely a lipid compound called a prostaglandin, increase the expression of two specific cellular membrane proteins on some immune cells. The interaction of these proteins, called programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1), leads to the suppression of an immune cell called helper T cell (Th1). This means that the cattle’s immune response is less able to combat invading tick-borne parasites.
Further investigation showed Asian blue tick saliva contains a high concentration of prostaglandin E2, which is known to induce PD-L1 expression. However future studies need to confirm if prostaglandin E2 plays a direct role in suppressing the cattle immune response. Also, since this study involved cells in the laboratory, the team says further research in live cattle is needed.
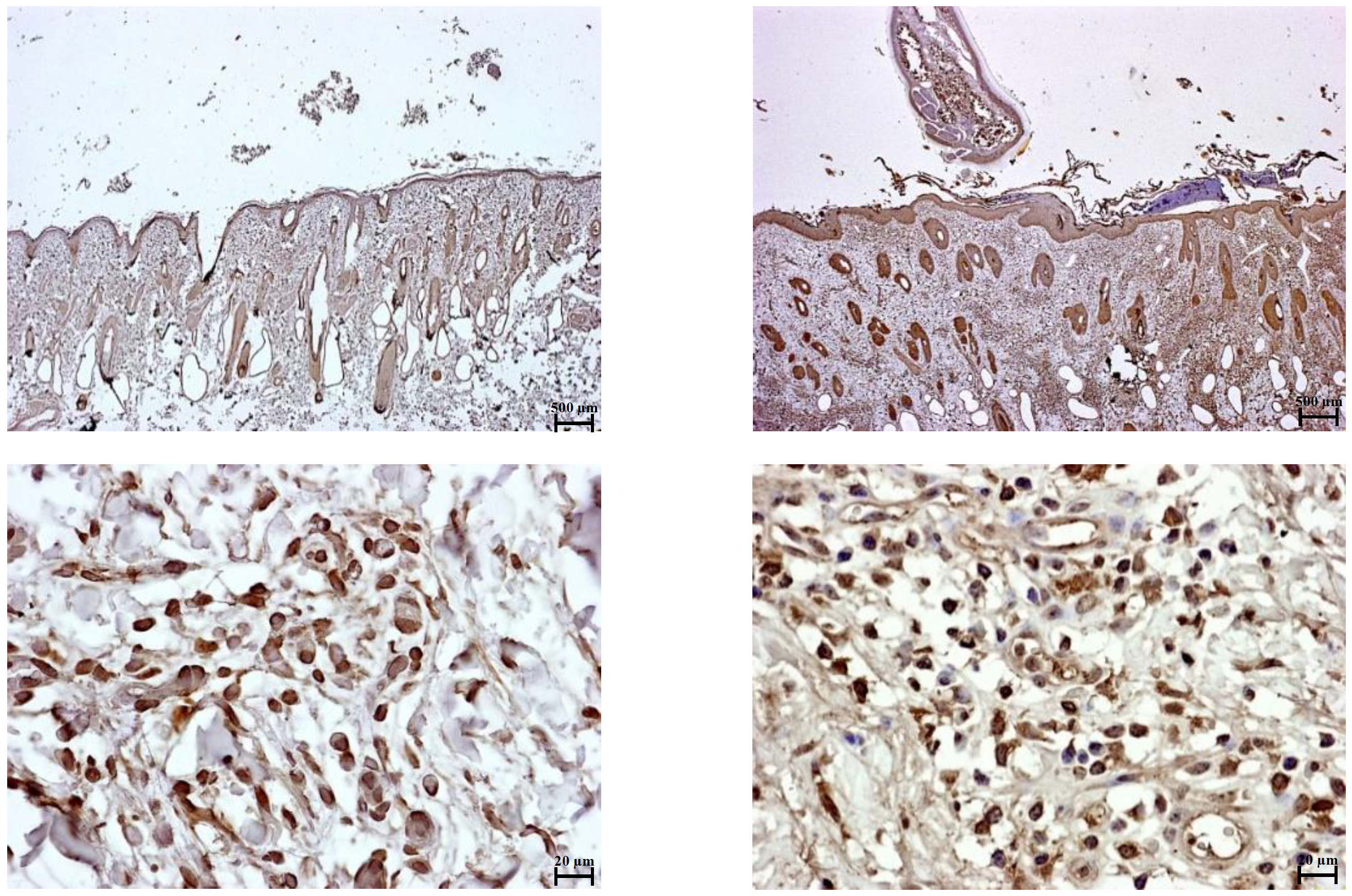
Immunohistochemical staining of PD-L1 in tick-nonattached (left) and tick-attached (right) sites on cattle. An increase in PD-L1 positive immune cells (visually, an increase in the amount and intensity of brown areas) was observed in tick-attached sites. (Yamato Sajiki, et al. Scientific Reports. January 13, 2021).
“Our findings suggest that Asian blue tick saliva inhibits the immune responses of helper T cells, at least in part, via the interaction between PD-1 and PD-L1,” says Konnai.
Associate Professor Satoru Konnai of the Laboratory of Infectious Diseases at Hokkaido University conducts research on the development of novel therapeutic strategy for intractable diseases control in animals; the pathogenesis of bovine leukemia; analysis of mechanism of tick-borne pathogen transmission and development of anti-tick vaccines.
The Portuguese press release from Universidade Federal do Rio Grande do Sul can be found here; the Portuguese press release from Universidade Federal do Rio de Janeiro can be found here.

Researchers from Hokkaido University, Universidade Federal do Rio Grande do Sul and Universidade Federal do Rio de Janeiro. Kei Watari (front row, maroon polo shirt), Yamato Sajiki (front row, dark blue polo shirt), Carlos Logullo (front row, blue checked shirt), Satoru Konnai (back row, white shirt) and Itabajara da Silva Vaz Jr (back row, orange T-shirt) were key contributors. (Photo: Universidade Federal do Rio Grande do Sul).
Original Article:
Yamato Sajiki, et al. Tick saliva-induced programmed death-1 and PD-ligand 1 and its related host immunosuppression. Scientific Reports. January 13, 2021.
DOI: 10.1038/s41598-020-80251-y
Funding:
This research was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (19KK0172); Japan Agency for Medical Research and Development (AMED; JP19fk0108068); the Project of the National Agriculture and Food Research Organization (NARO) – Bio-oriented Technology Research Advancement Institution (Research program on development of innovative technology, number 26058 BC; the special scheme project on regional developing strategy, grant 16817557); the JSPS and Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil, under the Japan-Brazil Research Cooperative Program (88881.153222/2017-01); and the National Council for Scientific and Technological Development (CNPq), Brazil (465678/2014-9, 405763/2018-2, 141395/2016-8).
Contacts:
Associate Professor Satoru Konnai
Laboratory of Infectious Diseases
Faculty of Veterinary Medicine
Hokkaido University
Tel: +81-11-706-5216
Email: konnai[at]vetmed.hokudai.ac.jp
Professor Itabajara da Silva Vaz Jr
Department of Veterinary Clinical Pathology
Universidade Federal do Rio Grande do Sul
Tel: +55-51-3308-6078
Email: itabajara.vaz[at]ufrgs.br
Professor Carlos Logullo
Leopoldo De Meis Institute of Medical Biochemistry
Universidade Federal do Rio de Janeiro
Tel: +55-21-3938-6786
Email: carloslogullo[at]yahoo.com.br
Sohail Keegan Pinto (International Public Relations Specialist)
Public Relations Division
Hokkaido University
Tel: +81-11-706-2185
Skype: hokudai.pr1
Email: en-press[at]general.hokudai.ac.jp
Assessoria de Imprensa
Universidade Federal do Rio Grande do Sul (UFRGS)
Tel: +55-51-3308-4008
Email: imprensa[at]ufrgs.br
Assessoria de Imprensa
Reitoria – Coordenadoria de Comunicação Social
Universidade Federal do Rio de Janeiro (UFRJ)
Email: press[at]ufrj.br
Related Press Releases:
The drug combination effective against bovine leukemia
Unraveling the immunopathogenesis of Johne’s disease
New therapeutic antibody for dog cancers
Overcoming immune suppression to fight against bovine leukemia

