Does society accept crops genetically modified without the introduction of transgenes? : A proposal for solving issues on regulations and labelling of genome edited crops
Research Press Release | February 27, 2015
| Press Release | ||
|---|---|---|
| Key Points |
・Our survey displayed that rice is currently the most frequently used crop for plant breeding by genome editing. Most of the rice mutants may be outside current regulations for genetically modified organisms (GMO). ・To encourage regulatory response, we propose four regulatory models for genome edited crops. ・We also propose a possible solution for social concerns about labelling of genome edited crops. |
|
| Overview | Modern genome editing technology has allowed for far more efficient gene modification, potentially impacting future agriculture. However, genome editing raises a regulatory issue by creating indistinct boundaries in GMO regulations because the advanced genetic engineering can, without introducing new genetic material, make a gene modification which is similar to a naturally occurring mutation. For open discussion, we have presented a general drawing on the regulatory issue*1.
An opinion article, to be published in the March issue of Trends in Plant Science, focuses on genome edited crops and considers solutions for the regulatory and social issues. In research on plant breeding by genome editing, the main target crop is rice, the staple food of the Japanese. However, most of the rice mutants may be out of the scope of current GMO regulations. To avoid health and environmental risks under unregulated situations, we built four regulatory models by classifying and summarizing various plant mutants produced by genome editing methods. Furthermore, we also propose a possible solution for social concern about labelling of genome edited crops. Genome editing technology is advancing rapidly; therefore it is timely to review the regulatory system for plant breeding by genome editing. Moreover, we need to clarify the differences between older genetic engineering techniques and modern genome editing, and shed light on various issues towards social acceptance of genome edited crops. |
|
| Outline |
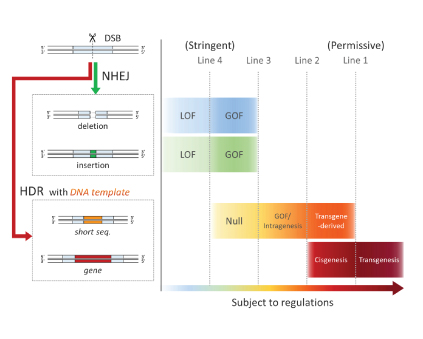
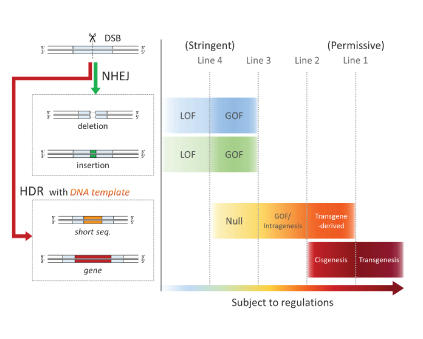
Background Genome editing technology, including ZFN, TALEN, and CRISPR/Cas*2, is bacterial nuclease-based genetic engineering and has facilitated various gene modifications even in higher organisms. Meanwhile, genome editing can produce genetically modified organisms which are extremely similar to naturally occurring mutants. For this reason, the advanced genetic engineering has raised regulatory issues under current GMO regulations which are generally based on a novel combination of genetic material, including the introduction of a transgene, thereby creating a GMO*3. We have presented a general drawing of the complicated regulatory issue in the process- and product-based GMO regulations and obtained many comments about the drawing. This study focused on genome edited crops, which are expected to be released to consumers soon, developed regulatory models by reflecting recent research reports, and considered a potential social issue. Methods 1. In-depth analysis of 13 articles in terms of the subject, gene modification efficiency, and type of modification. 2. Development of regulatory models linking with current GMO regulations. 3. Considering social issues that could occur when releasing genome edited crops to consumers. Results 1. Analysis of plant breeding by genome editing 13 studies of genetically modified varieties of barley, rice, sweet orange, wheat, soybean, corn, and tomato were analysed. The result showed that rice was the most frequently chosen (5/13) of all major crops. The most common modification types were mutations with a few bases deleted or inserted resulting in modified crops that resemble naturally occurring mutants (12/13). The genes targeted for mutation had been selected for the purposes such as introducing herbicide-resistance, increasing a nutrition value, changing a flavor, and more. Although gene modification efficiency varies between target genes, it reaches to 30-40% in some cases. Genome editing may induce unintentional mutations (off-target mutations) in plant genomes, which might be associated with health and environmental risks. However, less than half of the articles investigated the occurrence of off-target mutations(5/13). It was shown that genome editing can be used for crop breeding for various purposes. However, deletion or insertion mutants, which may be outside current GMO regulations, were frequently produced in the research reports. Therefore, the necessity of regulatory response to plant breeding by genome editing was confirmed. 2. Development of regulatory models for genome edited crops A regulatory concept with four potential regulatory lines was developed. Various plant mutants produced by genome editing were analyzed and categorized in terms of gene modification methods and functional changes, and mapped in order of relevance to the current product-based GMO regulations. Figure 1. Regulatory models for genome edited crops (simplified version) This analysis was performed on the premise that genome editing nucleases are introduced as RNAs or proteins, not DNA, and that no off-target mutations are induced. Mutants beyond a regulatory line are subject to the regulations. DSB; double-stranded break, NHEJ; non-homologous end-joining (gene modification without exogenous DNA), HDR; homology-directed repair (gene modification with exogenous DNA), LOF; Loss of function mutation, GOF; Gain of function mutation. Intragenesis; a process in which transferring a DNA sequence creates a new combination of gene elements derived from different genes within the cross-compatible species. Cisgenesis; a process in which an identical copy of a gene from a cross-compatible species (cisgene) is transferred to a related species. 3. Consideration of social issue In order to achieve social acceptance of genome edited crops, it is vital that the general public fully understands the safety and risks of genome edited crops. Moreover, it is also important to consider how to label food containing ingredients derived from genome edited crops because the civil movement,“Right-to-Know” to demand the labelling of GMOs is prevailing worldwide. A regulatory agency is likely to encounter difficulties in identifying small number of mutations (e.g. deletion or insertion of a few bases) when inspecting food containing genetically modified crops. If people want to know whether food contains ingredients of genome edited crops or not, a possible solution is the introduction of an additional DNA tag in genome edited crops. This solution can be an option to enhance the social acceptance as long as the DNA tag involves no health and environmental risks. |
|
| Conclusion |
This study showed that rice is the most frequently used crop in recent research on genome edited crops, and that most of the plant mutants may be outside the current GMO regulations. As appropriate regulatory response is needed, we built four regulatory models in order to resolve the indistinct regulatory boundaries which genome editing has created in GMO regulations. We propose that the most stringent regulation (Line 4 in Figure 1) should be initially adopted and gradually relaxed because the cultivation and food consumption of genome edited crops is likely to increase in the near future. As social acceptance of genome edited crops requires careful consideration of labelling of food containing genome edited crops, we propose a possible solution based on DNA tagging. Although regulatory response to genome editing is delayed worldwide, this study outcome should provide a basis for discussion on the regulations in the Ministry of the Environment as well as Hokkaido. Moreover, this article should also stimulate public discussion on how food containing genome edited crops should be labelled. |
|
| Perspectives | Genome editing can be used for various applications. However, not all the uses are socially accepted. Further discussion in each application (e.g. animal or silkworm breeding) is required with regard to ethical and social use of genome editing. | |
|
1 Hokkaido University PRESS RELEASE: Caution required for handling genome editing technology (2014/4/22) 2 ZFN: Zinc Finger Nuclease, TALEN: Transcription Activator-Like Effector Nuclease, CRISPR/Cas: Clustered Regularly Interspaced Short Palindromic Repeat/Cas 3 Basic concept of GMO regulations: According to the Cartagena Protocol on Biosafety, a ‘living modified organism’ (the technical legal term that is close to GMO) is stipulated as ‘any living organism that possesses a novel combination of genetic material obtained through the use of modern biotechnology’[http://bch.cbd.int/protocol/text/]. In Japan, GMOs are regulated under a product-based regulation, the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms (Cartagena Protocol domestic law). |
||
| Inquiries |
Tetsuya Ishii, Associate Professor, Office of Health and Safety, Hokkaido University TEL: +81-11-706-2126 FAX: +81-11-706-2295 E-mail: tishii@general.hokudai.ac.jp |
|
|
Japanese Link |
社会は遺伝子改変の痕跡がない作物を受け入れるか:ゲノム編集作物の規制と表示に関する提言 | |
| Publications | Towards social acceptance of plant breeding by genome-editing, Trends in Plant Science (2015.2.26) | |