Neural Signal Due to Pain Induction Develops Relapse of a Multiple Sclerosis Model!
Research Press Release | July 22, 2015
| Press Release | ||
|---|---|---|
| Key Points | ・Pain induction develops relapse of a multiple sclerosis1 model via neural signal2-mediated Gateway Reflex3.
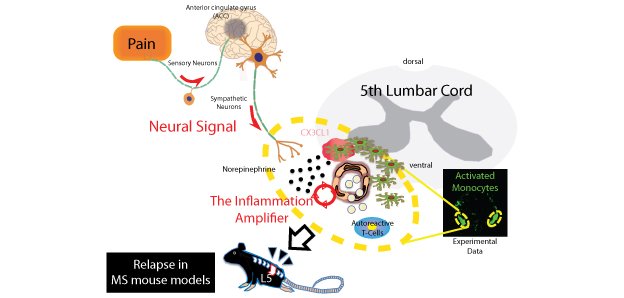
・Pain induction activates a sensory-sympathetic neural signal followed by a chemotactic factor-mediated accumulation of MHC class II+ activated monocytes that showed antigen-presentation activity at specific ventral vessels in the fifth lumbar cord of disease-recovered mice. ・A pain-mediated neural signal can be transformed into an inflammation reaction at specific vessels to induce disease relapse, thus making this signal a potential therapeutic target of many diseases of the central nervous system, including multiple sclerosis. |
|
| Overview | Pain is a symptom common to many diseases, and chronic pain greatly reduces the patient’s quality of life. Until now, however, pain was just considered to be a by-product of disease, not known to directly worsen the progress or symptoms of disease. In 2012, we reported on the phenomenon of the “Gateway Reflex” in which a neural signal, caused by the stimulation of muscles of the calves due to the earth’s gravity, causes the expression of a neurotransmitter around blood vessels of the fifth lumbar cord (L5) which leads to expression of a chemotactic factor that causes local lymphocytes accumulation, and then triggers pathogenesis in an animal model of multiple sclerosis, EAE4.
Our current research investigates how a neural signal due to “pain induction” affects disease symptoms. Our results indicate that if pain is experimentally inflicted at the onset of EAE then the EAE symptoms worsen, and conversely, if analgesics are administered, then symptoms improve. This shows that pain induction directly contributes to the progress of disease. Our next step used transfer EAE, which simulate the features of multiple sclerosis, to induce pain when the symptoms subsided (go into remission). We found that this caused the symptoms of EAE to relapse. Multiple sclerosis in humans repeatedly relapses, goes into remission, and is accompanied by pain. Our discovery suggests that it is the pain itself that triggers the relapse of multiple sclerosis. Since other types of stress did not lead to relapse, it is possible that pain induction triggers a neural signal. As our research progressed, we discovered a Gateway Reflex due to the new neural signal. A result of the excitation of sensory neurons that detect pain is that the region of the brain that governs the sensation of pain was activated, which in turn activated the sympathetic neurons that control the ventral blood vessels of the spinal cord. As a result, the neurotransmitter norepinephrine was released into the surrounding ventral blood vessels of the spinal cord so that the chemotactic factor CX3CL1 was expressed by vascular endothelial cells and activated monocytes, which remained in the central nervous system after the primary EAE. In the fifth lumbar cord (L5), which has excessively activated monocytes—most likely due to its position as the primary site of EAE—many of the activated monocytes locally accumulated, and as the result of the presentation of antigens, autoreactive T-cells in the periphery were activated, and caused the accumulation of various types of lymphocytes, which resulted in inflammation and the relapse of the disease. The accumulation of these lymphocytes required the activation of an “inflammation amplifier”5, which is a molecular mechanism for inducing excessive chemokines that we discovered in 2008. These results are a second example of Gateway Reflex-mediated inflammation development; and show that suppressing a neural signal caused by pain may be a new method to prevent the relapse of many diseases, including multiple sclerosis. Notes: 1 Multiple Sclerosis An autoimmune disease of the central nervous system. Depending on the location of the lesion in the central nervous system, the disease may appear, for example, as a visual disorder, motor impairment, or an urination disorder. It is estimated that the number of patients in Japan is about 10 persons out of 100,000. In 70% to 80% of patients there is repeated relapse and remission, accompanied by pain. In 2011 it was shown genetically that autoreactive T-cells, especially autoreactive helper T-cells, are involved in its pathogenesis. 2 Neural Signal A signal, which is an activated neural network between one organ and another organ (for example, from the soleus muscles to the dorsal blood vessels of the fifth lumbar cord, or, from pain to the dorsal blood vessels of the spinal cord), and that “locally” modifies the function of an organ. Due to a specific neural signal (an activated neural network), a neurotransmitter is released in the vicinity of the organ, and the function of that specific organ is modified. We discovered this in 2012 (Arima et al., Cell, 2012). Nerves run throughout the body, so if all the contents of neural signals are clarified, it may be possible to adjust the functions of organs throughout the body through artificial control. 3 Gateway Reflex A reflex in which norepinephrine is expressed near specific blood vessels due to a neural signal, and cells such as vascular endothelial cells react to it and express chemotactic factors, including chemokines, which creates a gateway through which lymphocytes can enter the specific organs, causing lymphocytes to locally accumulate and disrupt homeostasis, so that inflammation is triggered. This is a phenomenon that we were the first in the world to discover in 2012, and the original phenomenon involved “gravity-stimulated soleus muscles→sensory neurons→sympathetic neurons→dorsal blood vessels of the fifth lumbar cord→activation of the inflammation amplifier→inflammation of the fifth lumbar cord” (Arima et al., Cell, 2012). The present phenomenon, which involves “pain→sensory neurons→anterior cingulate gyrus in brain→sympathetic neurons→ventral blood vessels of the spinal cord→activation of the inflammation amplifier→inflammation of the fifth lumbar cord” is understood to be a second example of the Gateway Reflex. 4 EAE (Experimental Autoimmune Encephalomyelitis) An animal model of multiple sclerosis. By immunizing mice with endogenous proteins such as myelin oligodendrocyte glycoprotein, which is a component of the central nervous system, it is possible to induce symptoms similar to those of multiple sclerosis. It is also possible to induce the diseases by isolating helper T-cells from mice at the onset of EAE and transferring them into blood vessels of normal mice (transfer EAE). 5 Inflammation Amplifier A mechanism of inflammation induction in the non-immune cells such as vascular endothelial cells and fibroblasts. For example, if NF-κB and STAT, which are transcription factors, are simultaneously activated in non-immune cells by cytokine stimulations, then there is synergistic production of chemokines, which are factors that attract inflammatory cells. Various immune cells locally accumulate as a result of this over-production of chemokines, causing homeostasis to be disrupted at the site, inducing local inflammation (Ogura et al., Immunity, 2008). If the inflammation amplifier is chronically activated, then that leads to chronic inflammation, which is related to various diseases and disorders. We have already identified at least 1000 genes that exert positive control over the inflammation amplifier, and found that a significantly large percentage of them are genes related to human inflammatory diseases including autoimmune diseases, metabolic syndoromes, and neurodegenerative diseases (Murakami et al., Cell Reports, 2013). |
|
| Inquiries |
Masaaki MURAKAMI, Professor, Molecular Neuroimmunology, Institute for Genetic Medicine, Hokkaido University TEL: +81-11-706-5120 FAX: +81-11-706-7542 E-mail: murakami@igm.hokudai.ac.jp |
|
| Publications | A pain-mediated neural signal induces relapse in multiple sclerosis models, eLIFE (2015.7.20) | |
| References |
Arima Y et al., Regional Neural Activation Defines a Gateway for Autoreactive T Cells to Cross the Blood-Brain Barrier. Cell. 148: 447-457, 2012 Ogura H et al., Interleukin-17 Promotes Autoimmunity by Triggering a Positive-Feedback Loop via Interleukin-6 Induction. Immunity, 29: 628-636, 2008 Murakami Met al., Disease-Association Analysis of an Inflammation-Related Feedback Loop. Cell Reports. 3: 946-959, 2013 |
|
