New micro-platform reveals cancer cells’ natural behavior
Research Press Release | September 19, 2018
A new cell culture platform allows researchers to observe never-before-seen behaviors of live cancer cells under the microscope, leading to explanations of long-known cancer characteristics.
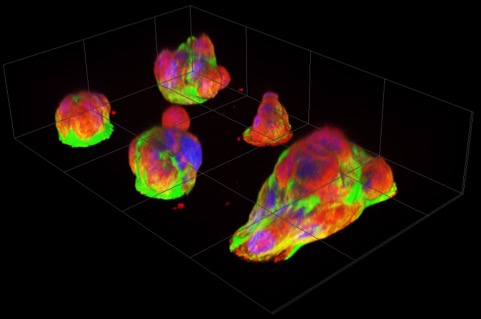
Fluorescence images of pancreatic cancer micro-tumors after overnight culturing. Papillary structures pile up on micro-attachment sites (diameter 30µm), with numerous cells visible per patch. The rightmost micro-tumor has extended over two attachment sites. Nuclei, actin filaments, and microtubules are labeled with blue, green and red fluorescent markers respectively. (Miyatake Y. et al., Scientific Reports, September 19, 2018)
The easy-to-produce platform developed by Hokkaido University researchers offers cancer cells micro-scale attachment sites that elicit never-before-seen behaviors highly relevant to cancer’s clinical properties. The observation of these behaviors shed light on the mechanisms behind well-known properties of pancreatic cancer, one of the most lethal malignant tumors, and may lead to the identification of new treatment targets.
“Cancer studies so far either use cell cultures in which cancer cells don’t necessarily behave naturally, or tissue samples that don’t allow live observation. So there is a big gap in our knowledge of how cancer cells actually behave,” says Assistant Professor Yukiko Miyatake, who led the study and focuses on cancer development mechanisms. To close this gap, she teamed up with Associate Professor Kaori Kuribayashi-Shigetomi who specializes on micro-nano-scale bio-engineering.
Together they created a new cell culture substrate from a coated glass slide with etched islands of 30µm diameter. For healthy cells, this is just enough space for one or two to attach. But when the researchers seeded them with pancreatic cancer cells (although they also tried other cancer cells with similar results) and incubated them overnight, the cells self-organized into micro-tumors that could move in a concerted way, as if it were one organism. Precursors to this turned out to be papillary structures that accommodate 4 or more cells by cell-in-cell invasion. This process, called entosis, is so far known only as a step in cell degradation. Here, the incorporated cells remained alive and, to their surprise, the incorporation was reversible.
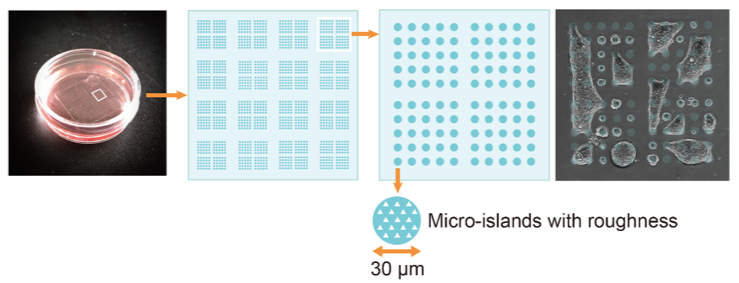
Micro-patterned plate used for the study. The dish has a diameter of 40mm, the square glass slide an edge length of 20mm. The picture on the right shows micro-tumors extending over multiple micro-attachment sites.
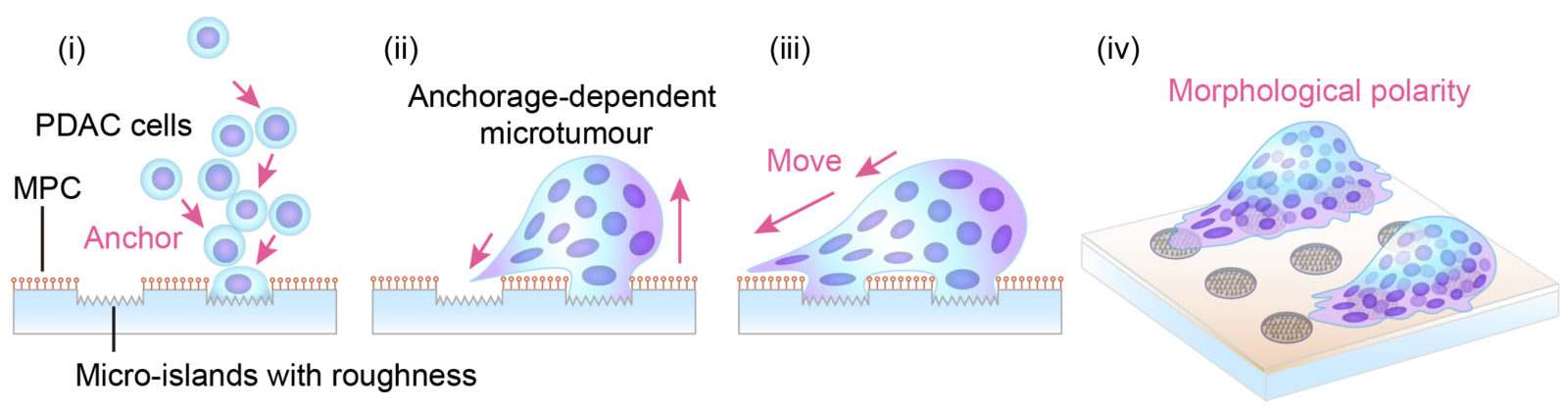
In the newly developed culture system, the pancreatic cancer cells (PDAC) self-organized and anchored to the micro-scale islands. Anchorage-dependent pancreatic cancer micro-tumors showed morphological polarity and motility. Arrows indicate presumed direction of movement. (Miyatake Y. et al., Scientific Reports, September 19, 2018)
When they treated the micro-tumors with the widely used anti-cancer agent Nocodazole, the micro-tumor disintegrated, but the now-detached cells survived. Moreover, the researchers observed the micro-tumors “fishing” for surrounding dead cells and ingesting them, in the process releasing chemical markers typical for dead cells. These markers ended up on the cancer cells’ surfaces, presumably masking them and enabling them to evade the immune system’s killer cells.
Striving to reduce the suffering cancer causes, Miyatake says: “I hope this easy and low-cost technique will find widespread adoption. If the discoveries made during these first observations are physiologically or pathologically relevant phenomena, many more new hints may be gleaned for the development of more effective cancer treatment approaches.”
Video created by the researchers summarizing the main observations resulting from culturing pancreatic tumor cells on the micro-patterned plate.
Original article:
Miyatake Y. et al., Visualising the dynamics of live pancreatic microtumours self-organised through cell-in-cell invasion, Scientific Reports, September 19, 2018.
DOI: 10.1038/s41598-018-32122-w
Funding information:
This work was supported by JSPS KAKENHI Grant Number 26460464, JP16H06280, and NPJ Grant Number NPS15058 by Nanotechnology Platform of MEXT, Japan.
Contacts:
Assistant Professor Yukiko Miyatake
Department of Pathology
Faculty and Graduate School of Medicine
Hokkaido University
Email: yukimiya[at]pop.med.hokudai.ac.jp
Associate Professor Kaori Kuribayashi-Shigetomi
Graduate School of Information Science and Technology
Hokkaido University
Email: kaorik[at]ist.hokudai.ac.jp
Naoki Namba (Media Officer)
Global Relations Office
Institute for International Collaboration
Hokkaido University
Tel: +81-11-706-2185
Email: pr[at]oia.hokudai.ac.jp
