Pain neuron-derived peptide prevents endotoxic death by targeting kynurenine pathway in microglia
Research Press Release | March 09, 2022
Joint release by National Institute for Physiological Sciences and Hokkaido University.
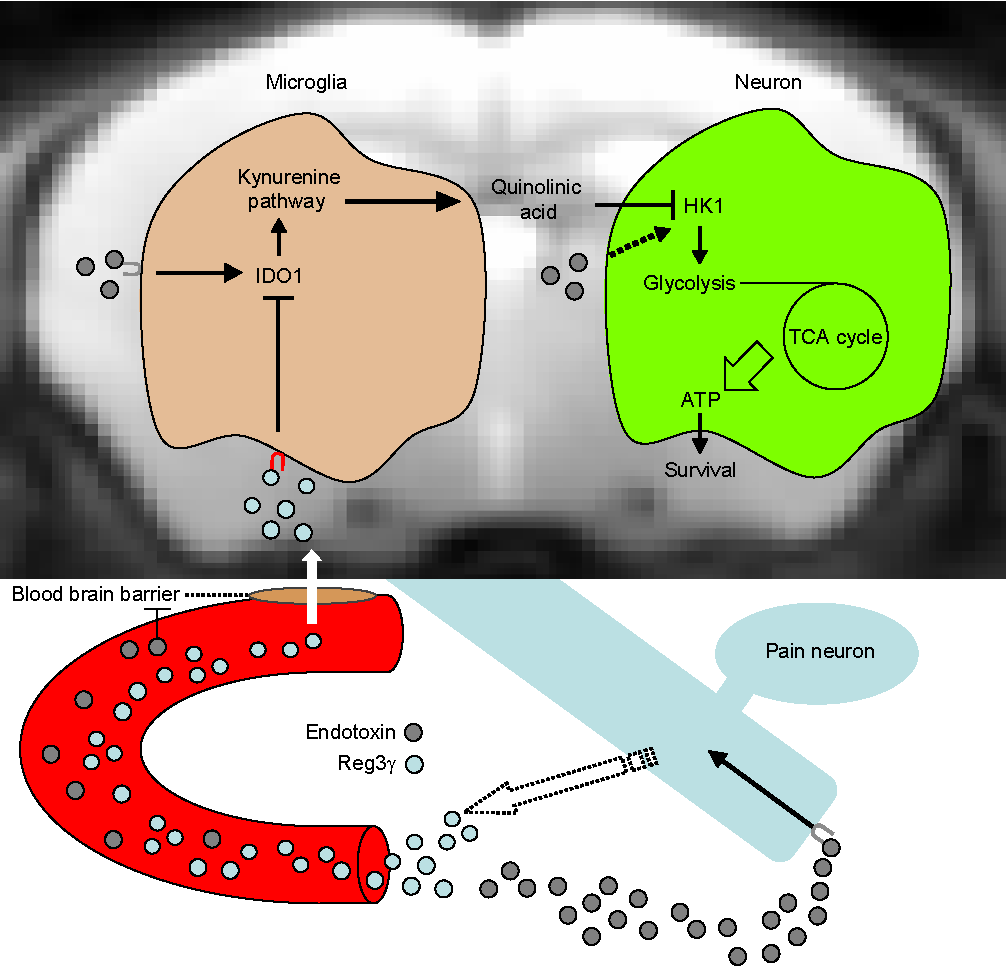
A research team led by the National Institute for Physiological Sciences and joined by Hokkaido University explored the role of pain neurons in the regulation of endotoxic death. They found that peptide named Reg3γ acts as a pain neuron-enriched brain-targeted hormone that protects the host from endotoxic death.
Sepsis is a high-mortality disease that arises when the body’s immune reaction to pathogens causes multi-organ defects. Although infection-induced pro-inflammatory cytokines are indispensable for pathogen elimination, dysregulated production of these factors can lead to endotoxic shock. Despite the extensive use of anti-TNF-α antibody administration or glucocorticoids in patients undergoing endotoxic shock, mortality rates remain high at 30%. These disappointing results suggest that the mechanism of endotoxic death is only partially explained by uncontrolled inflammation.
Studies of pain neurons have traditionally focused on the ion channels. Natural pain sensors such as the transient receptor potential cation channel subfamily V member 1 (TRPV1), and TRP ankyrin 1 (TRPA1) are expressed in afferent pain neurons and trigger pain to protect the organism from further harm. Emerging reports suggest that organ-innervated pain neurons and TRP channels also regulate dermal innate immunity, psoriasis, islet function, candidiasis, and osteomyelitis. Mechanistically, these phenomena are thought to be evoked by the paracrine secretion of nociceptor-derived peptides such as Calcitonin gene-related peptide (CGRP) and VIP, which modulate immune signals and endocrine pathways. These findings have also raised the question of whether pain neuron-derived “hormones” exist.
In this new study, Dr. Kenta Maruyama at NIPS, Assistant Professor Takeshi Kondo at Hokkaido University, and colleagues revealed that pain neuron-derived peptide Reg3γ penetrates the inflamed brain and suppresses the expression of microglial IDO1, a key enzyme of the kynurenine pathway. Endotoxin-administered pain neuron-null mice and pain neuron-specific Reg3γ deficient mice exhibit a high-mortality rate accompanied by decreased brain HK1 phosphorylation and ATP production despite normal inflammation. This metabolic arrest is only observed in the brain, and aberrant production of brain quinolinic acid, a neurotoxic metabolite of the kynurenine pathway, causes HK1 suppression. Notably, brain administration of Reg3γ protects mice from endotoxic death by enhancing brain ATP production. By identifying pain neuron-derived Reg3γ as a microglia-targeted hormone, this discovery provides novel insights into the understanding of tolerance to endotoxic death.
Original Article:
Erika Sugisawa, et al. Nociceptor-derived Reg3γ prevents endotoxic death by targeting kynurenine pathway in microglia. Cell Reports. March 08, 2022.
DOI: 10.1016/j.celrep.2022.110462
Funding:
This study was supported by a Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research, the Mitsubishi foundation; the Uehara Memorial Foundation; the Nakajima Foundation; the Fori Foundation; Tokyo Biochemical Research Foundation; Astellas Foundation for Research on Metabolic Disorders; Brain Science Foundation; and the Akaeda Medical Research Foundation.
Contacts:
Scientists
Kenta Maryama M.D., Ph. D.
Project Associate Professor
Division of Cell Signaling
National Institute for Physiological Sciences (NIPS)
Tel: +81-564-59-5294
Email: maruken [at] nips.ac.jp
Assistant Professor Takeshi Kondo
Department of Biochemistry
Faculty of Medicine
Hokkaido University
Tel: +81-11-706-5047
Email: tkondo[at]med.hokudai.ac.jp
Institutions
Akiko Nishio
Research Enhancement Strategy Office
National Institute for Physiological Sciences (NIPS)
Tel: +81-564-55-7723
email: pub-adm[at]nips.ac.jp
Sohail Keegan Pinto (International Public Relations Specialist)
Public Relations Division
Hokkaido University
Tel: +81-11-706-2185
Email: en-press[at]general.hokudai.ac.jp