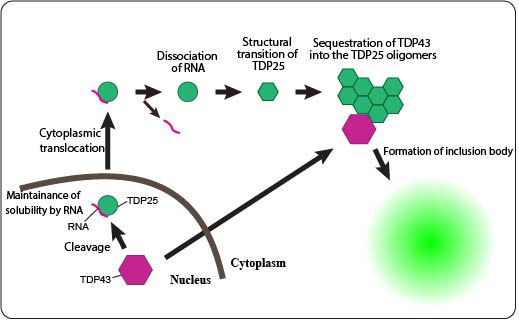
RNA suppresses protein aggregation and reduces neuronal cytotoxicity
Research Press Release | February 09, 2016
|
Elucidation of the mechanism of neuronal cell death in amyotrophic lateral sclerosis (ALS) |
||
|---|---|---|
|
Key Points |
|
|
|
Outline |
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease in which the motor neurons that give commands to muscles uniquely degenerate and are lost. In ALS, the protein named TDP431 is known to be a causative gene product2 that forms intracellular protein inclusion bodies3. It is also known that carboxyl-terminal fragments (CTFs) of TDP43 are found in the protein inclusion bodies within the motor neurons of ALS patients. In this research, by using a fluorescence imaging technique and fluorescence correlation spectroscopy4 with single-molecule sensitivity, in addition to discovering that TDP43 quickly translocated from the nucleus into the cytoplasm after cleaving, we also discovered that the formation of toxic aggregates of TDP25, which is one of the CTFs of TDP43, is suppressed by RNA. Furthermore, there are indications that these TDP25 aggregates exert cytotoxicity in the cytoplasm. These results have uncovered a new pathway for the formation of aggregates and inclusion bodies of proteins in ALS pathology. We believe that RNA is an important target in the elucidation of ALS pathology as well as in treatment to suppress ALS progression. All of this research was performed in the research laboratory of Hokkaido University Faculty of Advanced Life Science, Functional Cell Science (Professor Masataka KINJO), under Assistant Professor Akira KITAMURA, and has been published in the journal Scientific Reports. This research was performed with the assistance of a Japan Society for the Promotion of Science (JSPS) Grant-In-Aid for Scientific Research, category of Scientific Research C and Grant-in-Aid for Young Scientists B, and the Japan Agency for Medical Research and Development (AMED) Development of Advanced Measurement and Analysis Systems. |
|
| Terms |
|
|
| Inquiries |
Masataka KINJO, Professor Laboratory of Molecular Cell Dynamics, Faculty of Advanced Life Science, Hokkaido University E-mail: kinjo[at]sci.hokudai.ac.jp Akira KITAMURA, Assistant Professor Laboratory of Molecular Cell Dynamics, Faculty of Advanced Life Science, Hokkaido University E-mail: akita[at]sci.hokudai.ac.jp |
|
|
Japanese Link |
RNAがタンパク質の凝集を抑制し神経細胞毒性を低減する -筋萎縮性側索硬化症(ALS)の神経細胞死機構を解明- (01.14.2016) | |
|
Publications |
Interaction of RNA with a C-terminal fragment of the amyotrophic lateral sclerosis-associated TDP43 reduces cytotoxicity (1.13.2016) | |