Unlocking Carbon’s Mysteries
Research Highlight | June 03, 2021
Carbon is everywhere, in all aspects of our lives. Carbon is nature’s building block. It combines easily with carbon atoms and other atoms to make molecules that form everything. This process is called bonding.
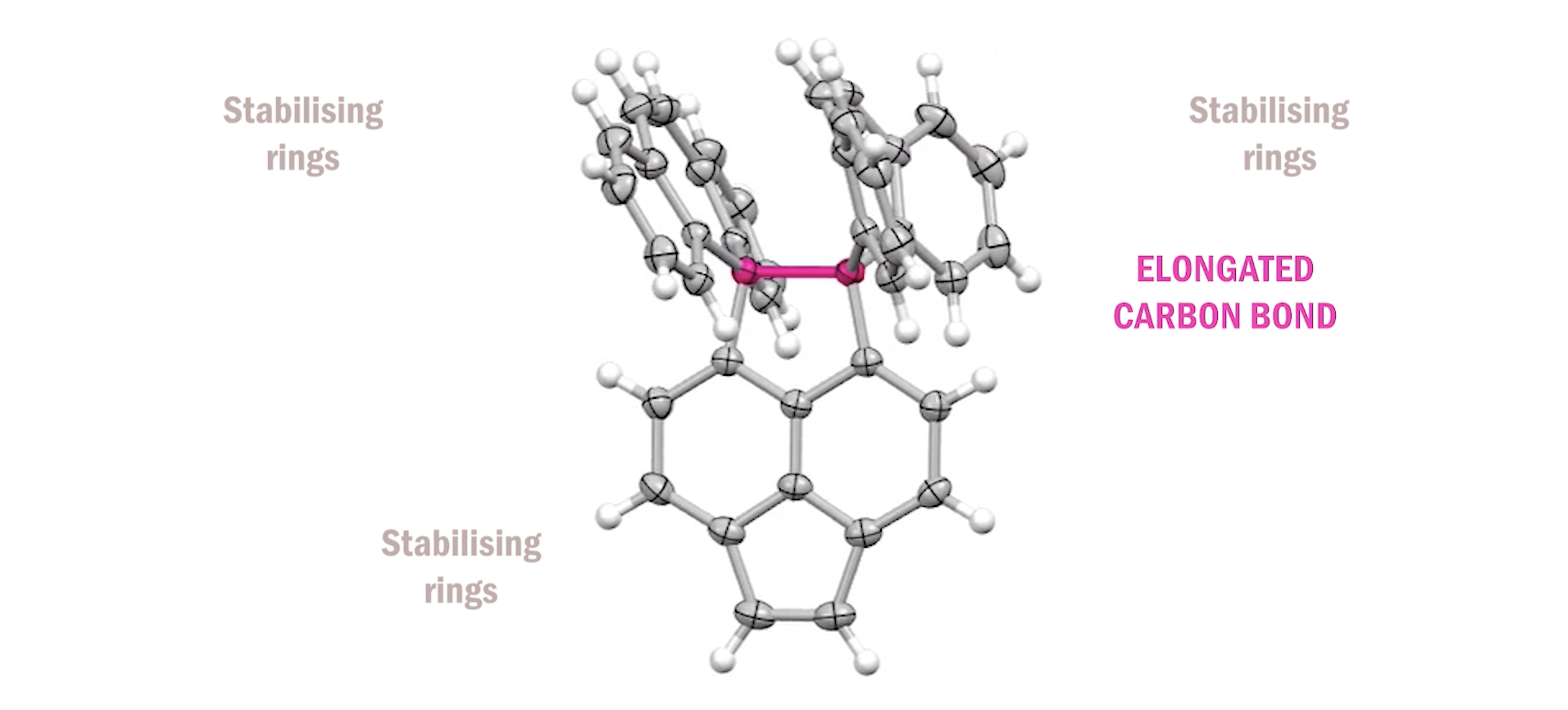
Associate Professor Yusuke Ishigaki and his team at Hokkaido University have been investigating elongation of carbon bonds. The single bond is known to be 1.54 angstroms long. The team succeeded in stretching it to 1.8 angstroms — 17% longer than the standard.
To do this the team created shell-like structures, enabling them to stretch the carbon bond while protecting it. They succeeded, proving the carbon-carbon bond is not rigid. Not only that, they set the world record with the longest carbon-carbon bond ever measured at 1.806 angstroms.
“Our group created the longest single bond, but we were also able to demonstrate that length was not it’s only feature,” said Associate Professor Yusuke Ishigaki.
Investigating further, the scientists found that a stretched carbon-carbon bond is weaker and more flexible than assumed. The stretched bond could further elongate or contract responding to light or heat. Such flexibility could give the molecule new properties like higher reactivity.
Future advancements may enable scientists to create chemicals that are less damaging to us and the environment, explore better ways to produce food, safer medicines, and much more.
“In the future, I aim to reach two angstroms in length, which is very likely to cause new phenomena,” Associate Professor Ishigaki concluded.
Written by Sohail Keegan Pinto
Related press releases:
Carbon-carbon covalent bonds far more flexible than presumed
New record set for carbon-carbon single bond length