Unlocking the secrets of nerve regeneration
Research Press Release | June 29, 2016
Hokkaido University researchers investigated what makes a specific nerve cell in the brain regenerate when others do not.
Nerves in the central nervous system of adult mammals do not usually regenerate when injured. The granule cell, a nerve cell located in the cerebellum, is different. When its fibres, called parallel fibres, are cut, rapid regeneration ensues and junctions with other neurons called “synapses” are rebuilt. The precise mechanism for this was unclear.
Researchers at Hokkaido University in Japan, together with colleagues from Sapporo Medical University School of Medicine and Niigata University, investigated the effect of a specific glutamate receptor, called GluD2, on parallel fibre regeneration.
GluD2 is a receptor located at the receiving end of the synapse, where the granule cell parallel fibres meet with another nerve cell type called the Purkinje cell, which is also present in the cerebellum. Nerve impulses pass via chemical mediators from one nerve fibre to another through synapses. The granule cell–Purkinje cell synapse, in particular, is where the regeneration occurs.
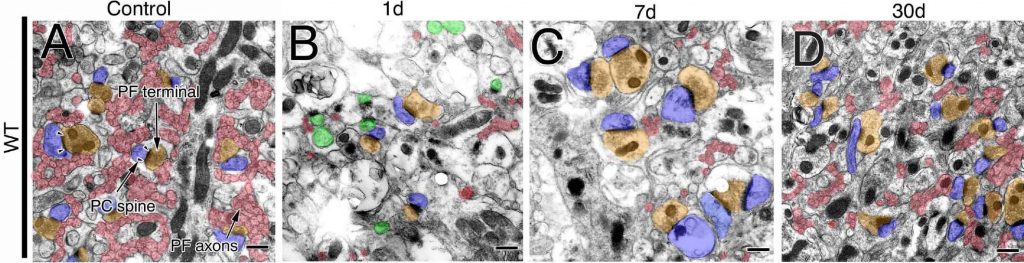 |
| Sections of the cerebellums after the incision. The number of parallel fibres (PF) was largely reduced on day 1. Parallel fibres were thickened and their terminals were enlarged on day 7. The number of parallel fibres and synapses was recovered on day 30. (Ichikawa R. et. al., The Journal of Neuroscience, April 27, 2016) |
The GluD2 receptor is involved in maintaining the synapses between granule cell parallel fibres and Purkinje cell nerve fibres. Shutting down the gene that encodes GluD2 results in a severe reduction in these synapses.
The team investigated the effects of cutting parallel fibres in normal mice and in mice that lack the GluD2 receptor. They examined serial sections of the cerebellum under a microscope, and reconstructed three-dimensional images of parallel fibre synapses on Purkinje cells one, seven and 30 days after the incisions were made.
They found that the cut parallel fibres in normal mice underwent three distinct phases. In the first “degeneration phase”, the number of parallel fibres and synapses were halved. In the second “hypertrophy phase”, the number of parallel fibres remained the same, but the team observed they had undergone thickening and there was enlargement of their terminals. Interestingly, each terminal tended to make multiple synapses as opposed to a single synapse under normal conditions. In the final “remodelling phase”, the number of parallel fibres was recovered and the thickening and enlargement that occurred during the hypertrophic phase were dissolved.
The parallel fibres in mice with no GluD2 receptor remained in the degenerative phase. This, the researchers conclude, indicates that GluD2 plays a “pivotal role” in the regenerative rewiring of parallel fibres.
The major obstacle to regeneration in other nerve fibres is the presence of inhibitory factors in the environment of the adult central nervous system, the researchers write in their study published in The Journal of Neuroscience. These inhibitors increase after injury. Further understanding of the role of GluD2 may allow researchers to unlock the regenerative capacity in the central nervous system.
Original article:
Ichikawa R. et. al., GluD2 Endows Parallel Fiber–Purkinje Cell Synapses with a High Regenerative Capacity. The Journal of Neuroscience, April 27, 2016
doi: 10.1523/JNEUROSCI.0161-16.2016
This work was supported by Grants-in-Aid for Scientific Research, Ministry of Education, Culture, Sports, Science and Technology, and also by the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from AMED, Japan.
Contacts:
Professor Masahiko WATANABE
watamasa[at]med.hokudai.ac.jp
Graduate School of Medicine,
Hokkaido University
Mr. Naoki NAMBA (Media Officer)
Global Relations Office
Office of International Affairs
Hokkaido University
Email: pr[at]oia.hokudai.ac.jp
Tel: +81-11-706-8034
