Water spin isomers hide their origin
Research Press Release | January 25, 2016
-
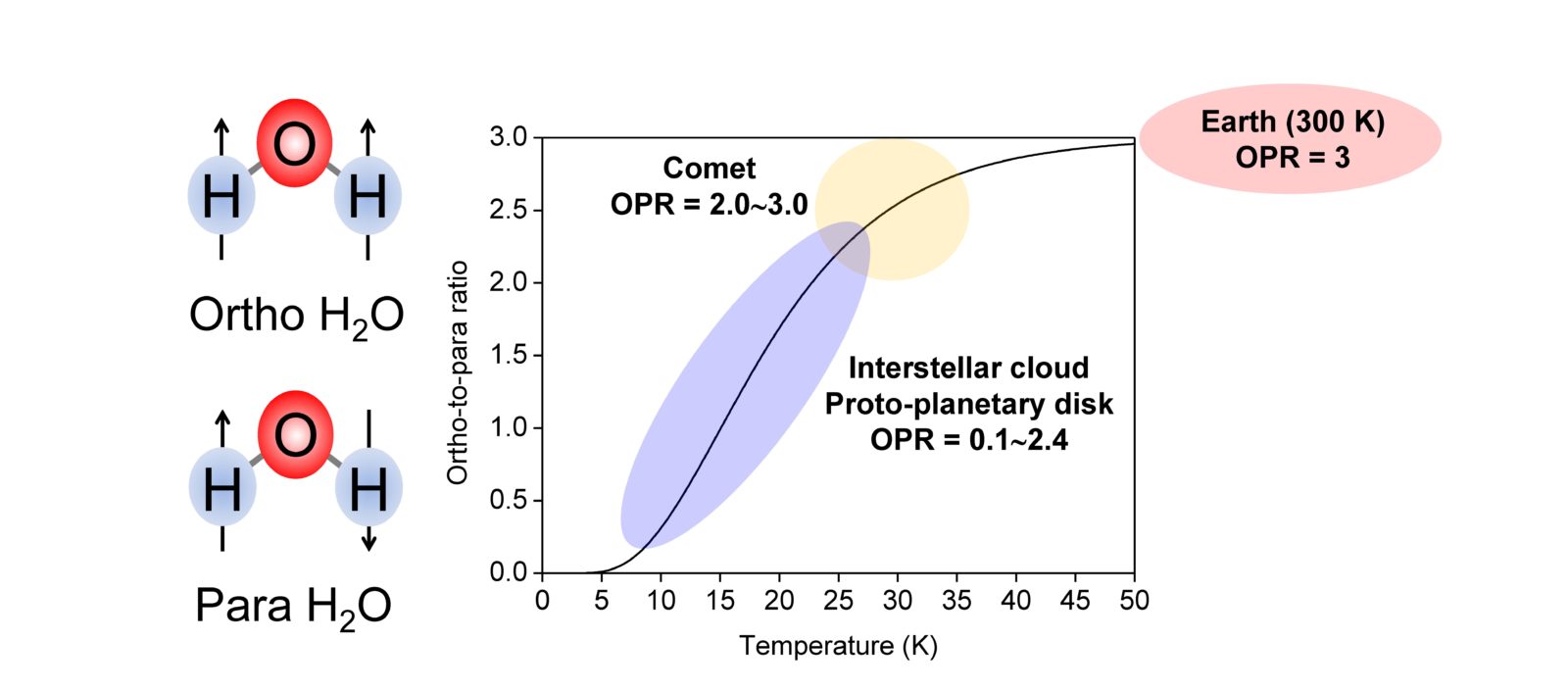 Fig. 1 Ortho-H2O and Para-H2O. At low temperatures below 50 K para-H2O becomes more stable than ortho-H2O, so the OPR changes from that under high temperature conditions (OPR = 3), and the amount of para-H2O increases.
Fig. 1 Ortho-H2O and Para-H2O. At low temperatures below 50 K para-H2O becomes more stable than ortho-H2O, so the OPR changes from that under high temperature conditions (OPR = 3), and the amount of para-H2O increases. -
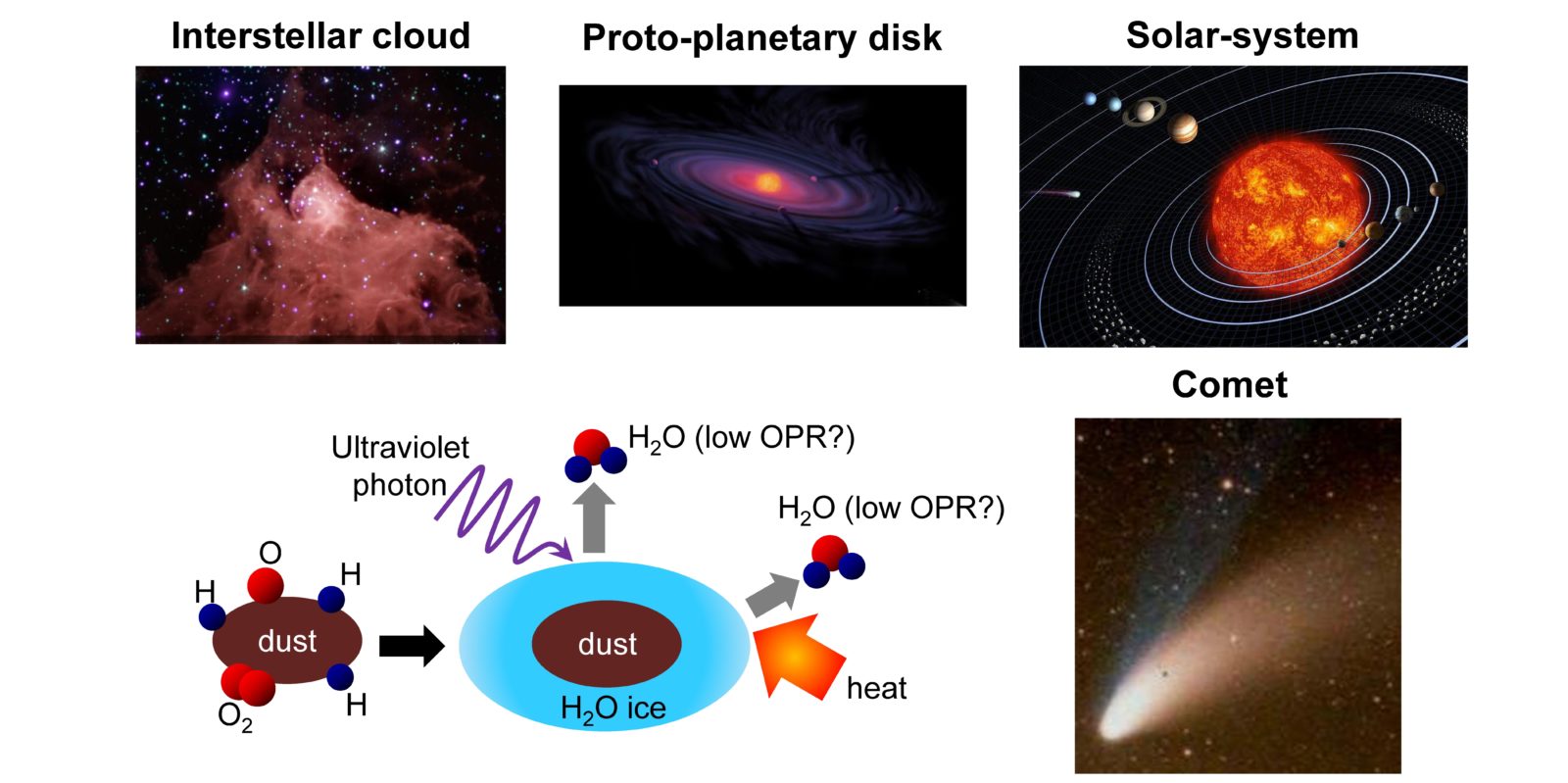 Fig. 2
Fig. 2
(top) Physical evolution of celestial bodies from interstellar cloud (star-forming region) to solar system
(bottom) Chemical evolution of substances accompanying the evolution of the celestial bodies.
Oxygen and hydrogen react on the surfaces of interstellar dust to form ice. It had been believed that, by observing the OPR of water that desorbs from the ice in interstellar dust and cometary nuclei we could learn the temperature at the time when the ice formed, but there was no experimental proof. -
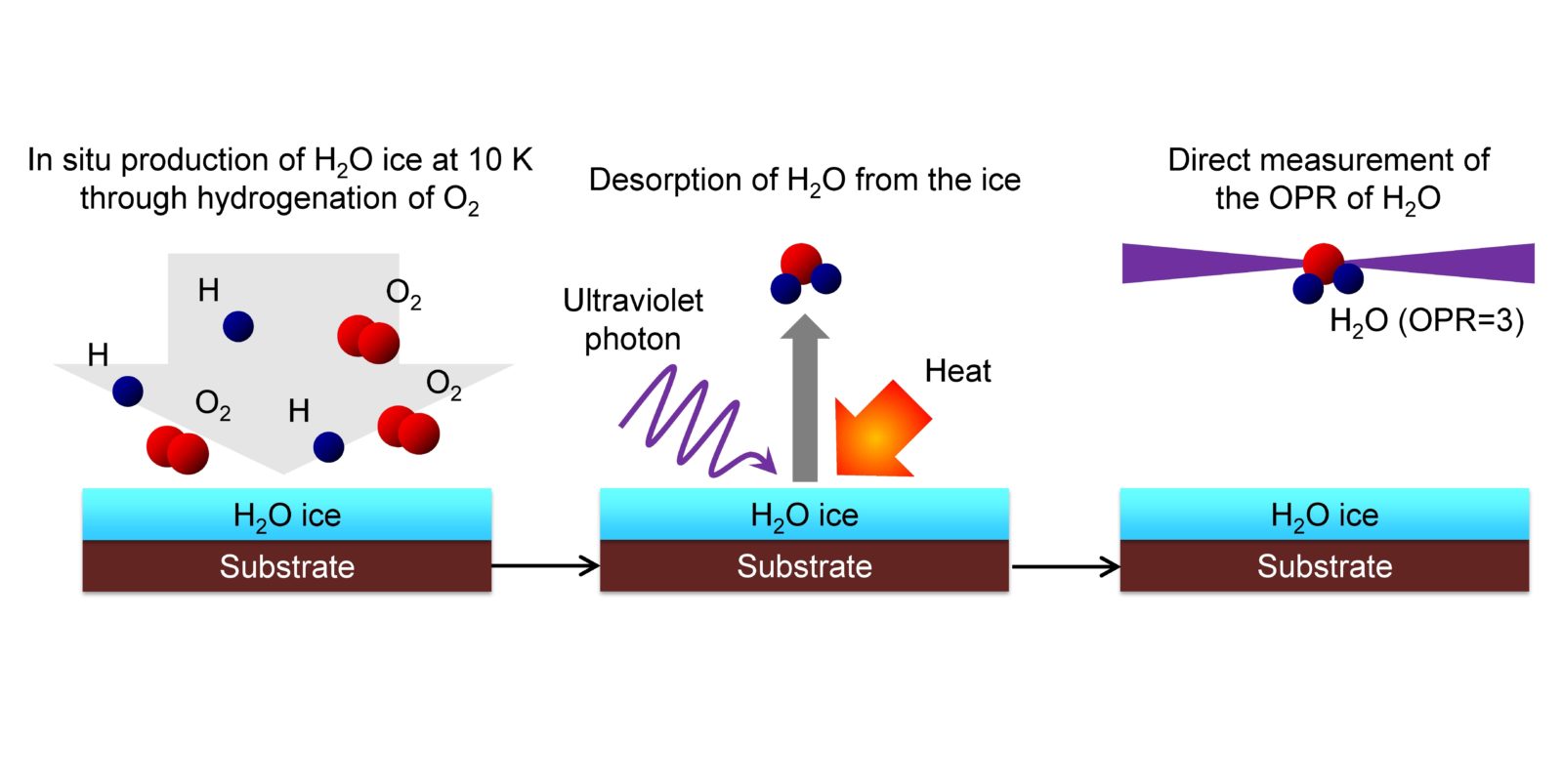 Fig. 3 Schematic diagram of the experiment. Ice is formed at -263°C (= 10 K) and the OPR of the water that desorbs from the ice is directly observed. As a result we learned that the OPR of the desorbed water did not reflect the temperature at the time of creation (OPR = 0.3) but rather it reflected the same high-temperature condition as that of the Earth (OPR = 3).
Fig. 3 Schematic diagram of the experiment. Ice is formed at -263°C (= 10 K) and the OPR of the water that desorbs from the ice is directly observed. As a result we learned that the OPR of the desorbed water did not reflect the temperature at the time of creation (OPR = 0.3) but rather it reflected the same high-temperature condition as that of the Earth (OPR = 3).
| Press Release | ||
|---|---|---|
| Key Points |
・Elucidation of the meaning of the anomalous ortho-to-para ratio (OPR)1 of water existing in comets and interstellar clouds, which has been an open question for many years. ・Discovery that it is not true that the OPR shows the temperature at the time when the water formed in space, as was previously thought. ・Proposal of a reinterpretation of past observational results, and a revision of the theory, regarding the origin of water in space and in the solar system. |
|
|
Background |
Water (H2O) exists ubiquitously in space such as in the interstellar clouds in which stars are born, in proto-planetary disks, in the comets and icy satellites of the solar system, and on the Earth. Therefore, water is a molecule that is related to evolution from interstellar clouds to the solar system. By studying the properties of water that have been created in space it is possible to understand the environment of the interstellar clouds and comets and to explore how the solar system formed. In particular, the ortho-to-para ratio (OPR) of water in space has recently received attention. Water (H2O) molecules can be classified into two types: ortho-H2O in which the spin directions of the nuclei of the hydrogen atoms are parallel, and para-H2O in which the directions are antiparallel (Fig. 1). At the temperature conditions of the Earth (27°C = 300 K), the OPR of H2O is 3:1 based on its quantum mechanical properties. However, at low temperatures below -223°C (= 50 K) the amount of para-H2O increases. It is known that the OPR of water in interstellar clouds and comets has more para-H2O than on Earth (Fig. 1). For example, on comets the OPR is 2.5, which corresponds to -243°C (= 30 K). Ortho-para conversion in the gas phase is extremely slow, so in astronomy it has been believed that the OPR of water in space is determined according to the time when the water was formed, so if the OPR is observed then one can learn about the temperature at the time when the water was formed in the past and therefore that the water in comets was formed in an environment of -243°C (= 30 K) 4.6 billion years ago. However, experiments to investigate whether the OPR really does show the temperature at the time that the water was formed had not been performed until now, because separately detecting ortho-H2O and para-H2O with high sensitivity is difficult. Consequently, the true meaning of the anomalous OPR of the water in space is unclear, despite the accumulation of observational studies spanning thirty years. |
|
|
Research Methods / Results |
In space, water forms first as ice through the chemical reaction of oxygen and hydrogen on the surfaces of small and extremely low-temperature dust grains called interstellar dust2 (Fig. 2). This ice-covered dust is then irradiated with strong light in interstellar clouds and proto-planetary disks, or is heated in comets, and the water is desorbed into a gaseous phase and its OPR can be observed. Our research has faithfully reproduced in the laboratory the process by which ice is formed in space and desorbed into a gaseous phase, and has directly measured the OPR using a method called resonance-enhanced multiphoton ionization spectroscopy3 (Fig. 3). Our result show that even if ice is formed at -263°C (= 10 K), the OPR of the water desorbed from the ice is not the OPR of 0.3 that corresponds to 10 K, but rather corresponds to the same high-temperature condition as that of the Earth (OPR = 3). In other words, we revealed that this water OPR does not show the temperature at the time of formation. |
|
|
Anticipated Outcomes |
Our research can greatly advance the understanding of how water formed during the evolution of the solar system from an interstellar cloud, and how water became incorporated in celestial bodies such as comets. For example, the hypothesis that comets formed 4.6 billion years ago at 30 K based on the OPR of cometary water is not correct, so a new theory is needed to understand the temperature conditions at the time when the solar system was formed. Also, we have learned that the chemical reactions that occur after water desorbs from ice are much more important than previously thought for explaining the origin of the anomalous OPR observed in space. Chemical reactions greatly depend on the physical environment (such as temperature and pressure) of a celestial body. Therefore in the future, by investigating the relationship between the OPR of water in space and the chemical reactions that occur at such sites, it will be possible to understand in detail the physical environments of celestial bodies. The results of this research will also be very helpful in correctly understanding the latest observational results that have been obtained, such as from the ALMA telescope and the Rosetta spacecraft. |
|
|
Terms |
1. Ortho-to-Para Ratio (OPR): The nuclei of hydrogen atoms that form water (H2O) molecules rotate like tops, and is called “spin.” H2O can be classified into two types: ortho-H2O in which the spin directions of the nuclei of the two hydrogen atoms are parallel, and para-H2O in which the directions are antiparallel, and they have different magnetic properties. The OPR of water on the Earth is 3:1. 2. Interstellar Dust: Fine particles of about 0.1 μm that exist in interstellar clouds. Their nuclei may contain minerals or carbonaceous materials, and their surfaces are covered with ice. This interstellar dust covered with ice is the raw material from which the bodies of the solar system (such as comets and planets) are formed. 3. Resonance-Enhanced Multiphoton Ionization Spectroscopy: When molecules are irradiated with concentrated laser light the molecules are ionized and can be detected as an electrical signal (photoionization spectroscopy). In the usual photoionization spectroscopy it is impossible to separately detect ortho-H2O and para-H2O. However, by adjusting laser light to a specific wavelength and using resonance-enhanced multiphoton ionization spectroscopy, it is possible to selectively ionize and perform high-sensitivity detection of just ortho-H2O, or of just para-H2O. |
|
| Inquiries |
Astrophysical Chemistry and Ice and Planetary Science Group, Institute of Low Temperature Science, Hokkaido University Assist. Prof. Tetsuya HAMA TEL:+81-11-706-5474 FAX:+81-11-706-7142 hama[at]lowtem.hokudai.ac.jp |
|
|
Japanese Link |
宇宙の水の異常なオルト:パラ比の意味を解明 ~宇宙・太陽系の水の起源の定説を覆す~ (01.04.2016) | |
| Publications | Statistical ortho-to-para ratio of water desorbed from ice at 10 kelvin, Science, (01.01.2016) | |
