Why don’t fish freeze to death in icy water?
Research Press Release | April 18, 2017
In the microgravity experiments at the International Space Station (ISS), scientists revealed that supercooled water containing antifreeze glycoproteins accelerates and oscillates its ice crystal growth rate. This seemingly contradictory result may lead to a better understanding of the mysterious antifreeze effect in living organisms.

Fish can survive even in subzero environments, such as under ice floes. Researchers have hypothesized that when glycoproteins contained in fish blood are absorbed on the surface of ice crystals, it curbs the growth of ice crystals. Verifying the functions of these glycoproteins requires precise measurements of the normal growth rates of crystals over time. Yet this is difficult to do so on the Earth because of the natural convective flow around the growing crystal induced by gravity.
The researchers, led by Hokkaido University Professor Emeritus Yoshinori Furukawa, hoped to use the microgravity conditions of space to accurately measure the normal growth rates of crystal faces, as convective flow does not occur in this environment.
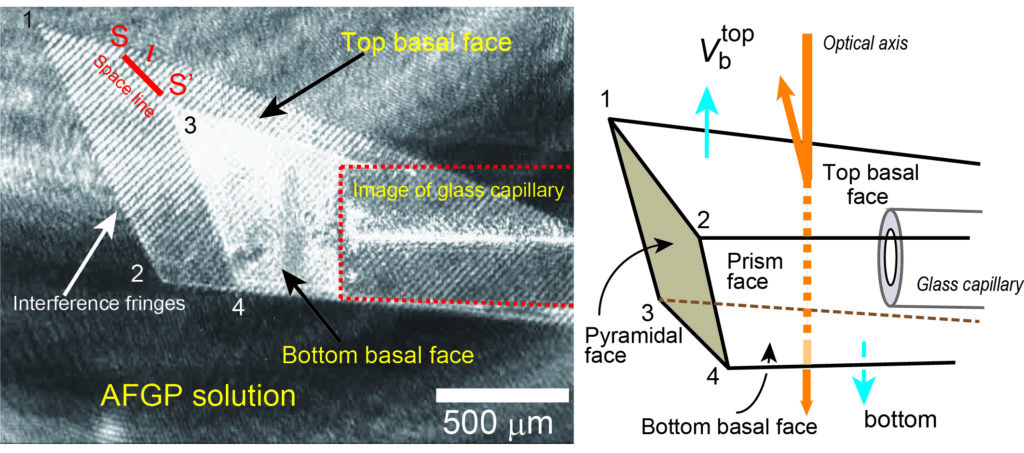
To carry out the experiments on the ISS, Hokkaido University’s Institute of Low Temperature Science and JAXA jointly developed Ice Crystal Cell 2, a device for measuring the speed of ice crystal growth in space. Once installed in the Japanese experiment module KIBO, experiments were carried out by controlling the device using signals from the ground.
The researchers conducted 124 experiments of which 22 were deemed to have accurately measured ice crystal growth rates in supercooled water containing a glycoprotein impurity. The results showed that the bottom basal faces of the ice crystals grew three to five times faster than in pure water. Ice crystals also exhibited periodic oscillations as they grew. “The results were contrary to what was expected, as the glycoprotein actually facilitated the growth of ice crystals, rather than curbing it,” says Ken Nagashima of the research team.
What, then, explains glycoprotein’s antifreeze effect? The researchers discovered the tricky process in which flat crystal faces with high growth rates were truncated by faces with slower growth rates, causing the polyhedral crystal to be surrounded by only flat faces with the lowest growth rates. This resulted in greatly slowing down the growth of the ice crystals.
“Our results suggest that the prevention of freezing in living organisms cannot be solely explained by the growth depression effect of glycoproteins. In other words, the novel mechanism we observed is essential for preventing living organisms from freezing,” says Nagashima. “The function of glycoproteins in ice crystal growth is closely connected to how biopolymers regulate the growth of various inorganic crystals. A better understanding of this may lead to the creation of novel materials”, he added.
Original article:
Furukawa Y. et al., Oscillations and accelerations of ice crystal growth rates in microgravity in presence of antifreeze glycoprotein impurity in supercooled water, Scientific Reports, March 6, 2017.
DOI:10.1038/srep43157
Contacts:
Assistant Professor Ken Nagashima
Institute of Low Temperature Science
Hokkaido University
Email: nagasima[at]lowtem.hokudai.ac.jp
Naoki Namba (Media Officer)
Global Relations Office
Institute for International Collaboration
Hokkaido University
Tel: +81-11-706-8034
Email: pr[at]oia.hokudai.ac.jp
