TRIM29 regulates the assembly of DNA repair proteins into damaged chromatin
Research Press Release | June 29, 2015
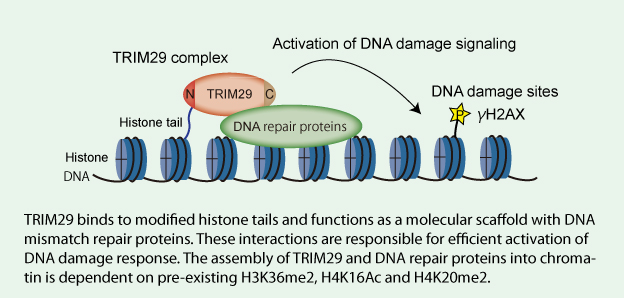
Model for TRIM29 function in activation of DNA damage signaling
| Press Release | ||
|---|---|---|
| Key Points | Although DNA double-strand break repair is mediated by numerous proteins accumulated at DSB sites, how DNA repair proteins are assembled into damaged chromatin has not been fully elucidated. Here, we show that a member of the tripartite motif protein family, TRIM29, is a histone-binding protein responsible for DNA damage response (DDR). | |
| Overview | We found that TRIM29 interacts with BRCA1-associated surveillance complex, cohesion, DNA-PKcs and components of TIP60 complex. The dynamics of the TRIM29-containing complex on H2AX nucleosomes is coordinated by crosstalk between histone modifications. TRIM29 binds to modified histone H3 and H4 tails in the context of nucleosomes. Furthermore, chromatin binding of TRIM29 is required for the phosphorylation of H2AX and cell viability in response to ionizing radiation. Our results suggest that TRIM29 functions as a scaffold protein to assemble DNA repair proteins into chromatin followed by efficient activation of DDR. | |
| Inquiries |
Shigetsugu HATAKEYAMA, Professor, Department of Biochemistry, Graduate School of Medicine, Hokkaido University TEL: +81-11-706-5047 FAX: +81-11-706-5169 E-mail: hatas@med.hokudai.ac.jp |
|
|
Japanese Link |
ゲノム DNA の修復を制御する分子の機能解明 | |
| Publications | TRIM29 regulates the assembly of DNA repair proteins into damaged chromatin, Nature Communications (2015.6.22) | |
