Forecasting biological damage caused by proton therapy
Research Highlight | November 06, 2020
This article is an excerpt from the research magazine “Tackling Global Issues vol.2 New Era of Radiation Therapy to Fight Cancer.” Click here to see the table of contents.
Taeko Matsuura works with medical professionals to make proton therapy more accurate and efficient, rendering expertise from the perspective of a physicist. While developing an algorithm to evaluate the biological effects on tumors and healthy tissues, she ventures into the domain of ultrasonic waves to verify proton beam ranges.

Dr. Taeko Matsuura, Associate Professor of Quantum Science and Engineering at Hokkaido University, Medical Physicist.
When she was a graduate student conducting research on basic particle theories, Associate Professor Taeko Matsuura’s main interests lay in finding “what we would see if we reached the end of the universe” and gleaning a better understanding of spacetime. But some soul-searching while she was a postdoctoral researcher prompted Matsuura to say goodbye to fundamental physics and venture into applied physics in the medical domain, which was also a subject of interest as a student.
Matsuura came to Hokkaido University in 2010 after a stint at the National Cancer Center Hospital East, which was the first hospital-based institution to conduct proton therapy in Japan. Since arriving in Hokkaido, Matsuura has harnessed her expertise in physics to conduct research toward improving proton beam therapy for Hokkaido University’s Proton Beam Therapy Center, a facility that opened a few years after her arrival.
“I started with several staff members at a medical physics lab for proton beam therapy, but later like-minded researchers joined me to develop hardware and a simulator to improve the therapy from a physical point of view,” Matsuura said. “My priority is to make proton beam therapy more accurate and efficient.” She has contributed to realizing effective proton therapy with reduced side effects by developing a method to calculate the appropriate dose for each patient and a simulator to estimate the actual dose delivered. She also developed a device that spreads out a sharp peak of proton beam so it can be delivered to a wider area at once.
Algorithm developed to gauge biological effects
Now Matsuura is tackling one of the hottest topics in proton beam therapy: evaluating and assessing the biological effects of proton beams. “Calculated dose distribution is not the same as the actual biological damage done to the tumor and adjacent healthy tissues,” Matsuura explained. “Biological damage is done off the path of a proton beam, and we sometimes find that healthy tissues or an organ a few millimeters beyond where the beam was supposed to stop have been damaged.”
To gauge the actual biological damage, Matsuura and her team developed a novel calculation algorithm for linear energy transfer (LET), the amount of energy that protons transfer to tissues. Biological damage is proportional to LET: The higher the LET, the greater the damage.
The algorithm Matsuura’s team developed is called the “Dual-LET kernel model” and enables a quick and accurate calculation of LET. “We can calculate LET in a few minutes, which makes it possible to use the model in a clinical routine,” Matsuura said. This development represents a leap forward in LET calculation. The conventional Monte Carlo simulation takes more than 10 hours to complete the calculation and another available algorithm, called the “Single-LET kernel model,” has been dismissed as impractical because of its extreme inaccuracy.
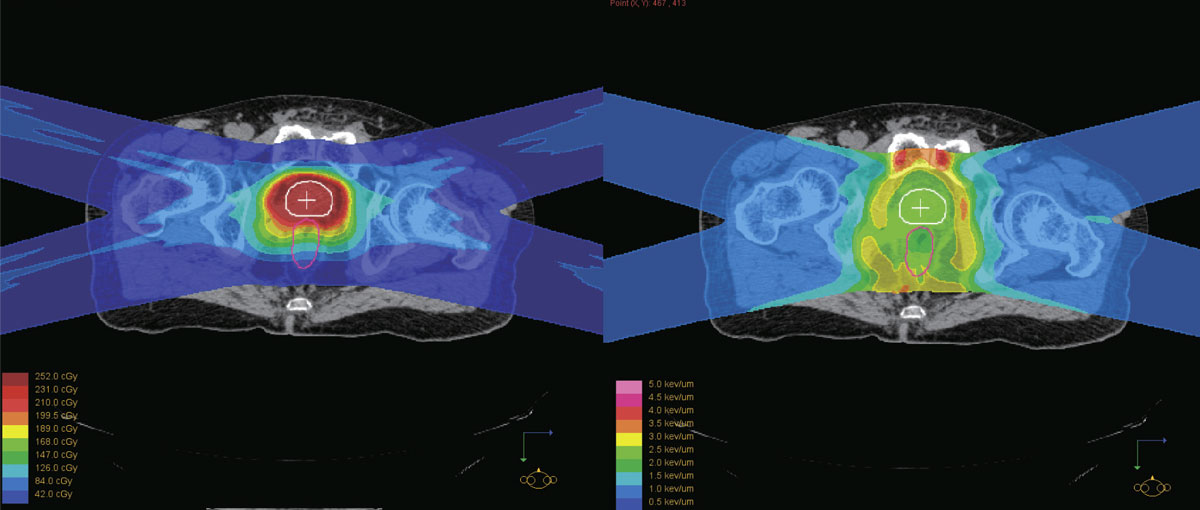
The physical dose distribution of a proton beam (left) is not the same as LET distribution which represents the actual biological damage (right). The team’s Dual-LET kernel model enables quick calculation of LET while delivering accurate results.
Calculating LET is necessary not only to reduce side effects, but also for killing the tumor effectively. “Even though the same dose is given, the magnitude of LET varies. If LET is low, damage is done to only sporadic areas, failing to break strands of the DNA double helix of cancerous cells and thus failing to kill them,” Matsuura said. “If that occurs, cancerous cells can quickly recover and proliferate again.” She hopes the algorithm will eventually be used in clinical routines, but several hurdles remain, such as issues in legality and cost. Matsuura said these problems will be addressed gradually.
Ultrasonic waves used to verify proton beam range
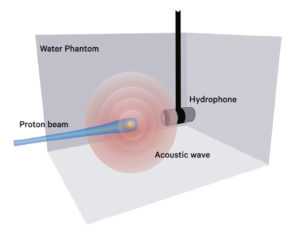
Looking ahead, Matsuura is involved in developing a real-time verification method for a proton beam range by using ultrasonic waves. She has teamed up with a Turkish expert in ultrasonic waves who is a researcher at the university as part of the university’s Global Institution for Collaborative Research and Education (GI-CoRE) project.
“At present, we can’t see exactly where proton beams are delivered to a patient lying on the treatment room bed right in front of us,” Matsuura said. “Ultrasonic waves could be a solution for finding the exact range of proton beams.”
When proton beams are delivered to a spherical metal marker embedded in a tumor, it generates a single frequency (resonance) acoustic wave, or a shock wave from a pulsed ion beam. This acoustic wave could be used to verify the proton beam’s range, or to pinpoint in real-time where the beam reached the Bragg Peak in relation to the gold marker, according to Matsuura’s research plan. Although full-fledged research has yet to start, Matsuura has high hopes that the method will become an indispensable tool in the treatment room in the future. “If we are able to know instantly where and when the beam reaches its Bragg Peak, we will all have peace of mind about the treatment, enabling us to provide better care to patients,” Matsuura said.
Click here to see the table of contents.