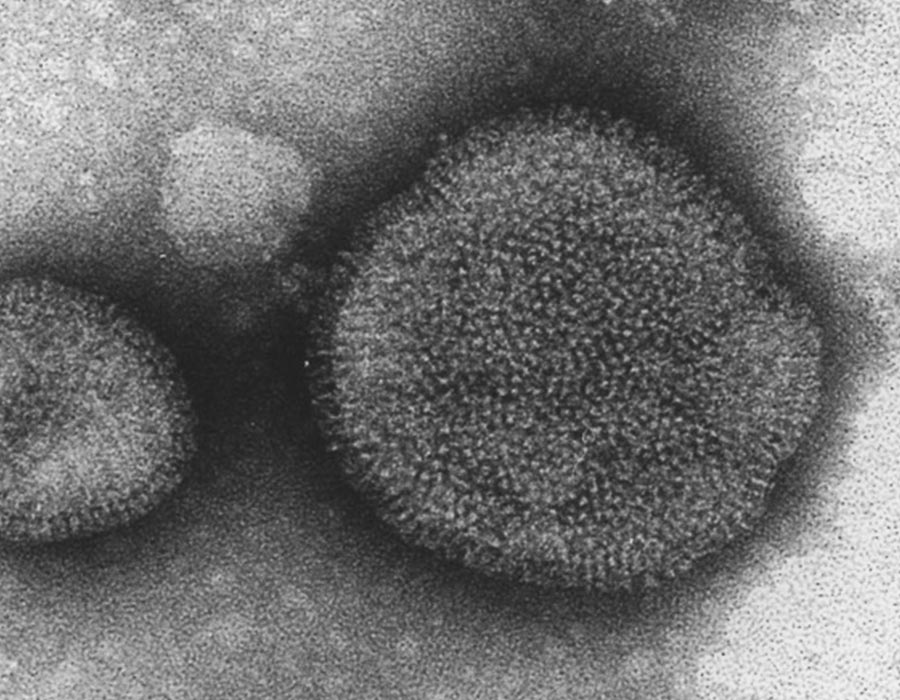
Influenza expert with unbending faith in research
Research Highlight | December 24, 2020
This article is an excerpt from the research magazine “Tackling Global Issues vol.3 Fighting the menace of zoonosis.” Click here to see the table of contents. For the Japanese translation, click here.

Hiroshi Kida, DVM, Ph.D.
University Professor, Hokkaido University
Head, Specially Invited Professor of the Research Center for Zoonosis Control (CZC)
Head, Collaborating Research Center for the Control and Prevention of Infectious Diseases, Nagasaki University
Head of the WHO Collaborating Centre for Zoonoses Control
Hiroshi Kida, a top authority on zoonoses, has frequently been challenged by naysayers who dispute virtually everything he has asserted about avian, pandemic and seasonal influenzas.
But every time he faced such a challenge, Kida simply concentrated on his pursuit of scientific truths involving influenza – one of the zoonoses – rather than engaging in verbal arguments. “In some cases, it was me against 100 opponents,” Kida recalled. “But influenza can be tackled only by steadily doing careful research. In 10 years, we all will know where the truth lies.”
One recent assertion Kida made is that inactivated whole virus particle vaccines, which are now undergoing clinical studies, should replace conventional split vaccines made of viruses that are disrupted by ether or detergent.
Kida is a long-time proponent of using whole virus particle vaccines after he succeeded in making one as a researcher at Takeda Pharmaceutical Co., Ltd. in the 1970s. Whole virus particle vaccines are more immunogenic than split vaccines, he claims.
“In current clinical trials, whole virus particle vaccines have shown they are effective,” Kida said. “They are also much cheaper to produce because there is no need to disrupt the viruses.”
Since 2015, Kida has been working with all five influenza vaccine producers in Japan to study the introduction of inactivated whole virus particle vaccines. The National Institute of Infectious Diseases in Japan has joined this industry-academia-government joint project, while the Japan Agency for Medical Research and Development has provided much-needed funds for the research. “Failure is not an option,” Kida said, expressing his resolve to make the vaccines available to society.
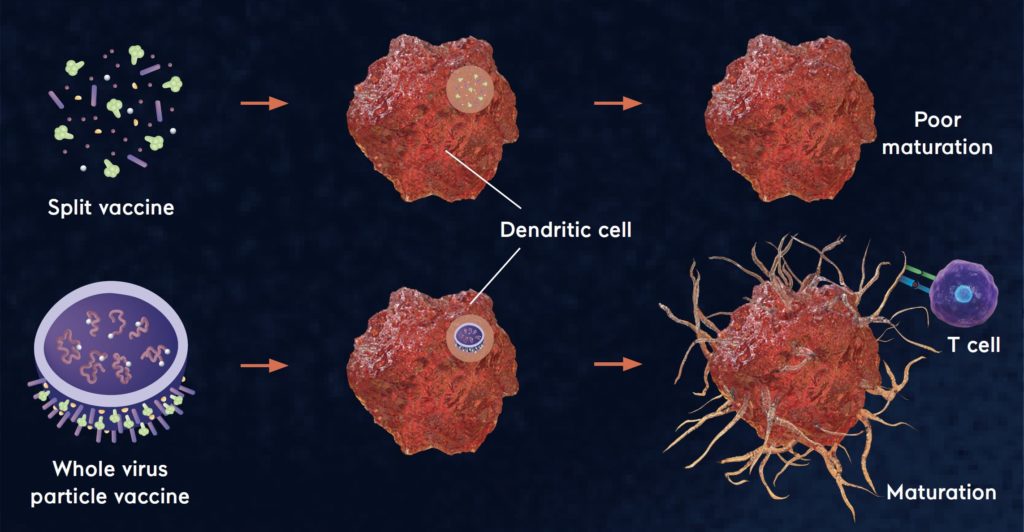
Antigen presentation: When exposed to a split vaccine (SV), a dendric cell (DC) – a special type of immune cell – displays a poor maturation and antigen presentation (top) while it exhibits a strong maturation and efficient antigen presentation when exposed to whole virus particle vaccines (bottom).
Identifying natural reservoirs and transmission routes of avian influenza
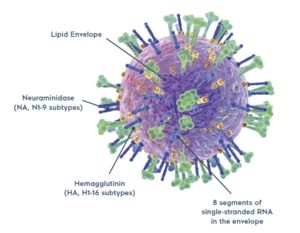
The virus has hemagglutinin (HA) and neuraminidase (NA) proteins on its surface. HA has sixteen subtypes and NA has nine. (Illustration Adobe Stock © Axel Kock)
After a seven-year stint at Takeda, Kida joined Hokkaido University in 1976 to pursue his research on influenza. Kida later established that ducks are the natural host for Type A influenza viruses and identified the transmission route to humans for the first time.
The migratory bird is orally infected with the virus with varying hemagglutinin (HA; a glycoprotein) and neuraminidase (NA; enzyme) subtypes as they nest in lakes and marshes in Siberia, Canada or Alaska near the Arctic in summer. The virus proliferates in the duck’s colon crypts and is discharged with feces, but does not do any harm to the duck. When the duck migrates south in autumn, it infects poultry and livestock on the way to or in its wintering places, such as southern China, Southeast Asia, the southern United States and Mexico. The virus is transmitted by water to domestic ducks and geese and then to terrestrial poultry such as quails, which serve as intermediate hosts that transmit the virus to chickens. While water birds have the receptor to viruses in their intestine, land birds such as chickens have the receptor in their respiratory organs. The infection of quails makes it easier for the virus to spread to chickens.
As the virus is repeatedly transmitted between chickens over more than six months, it can acquire pathogenicity in chickens. Only H5 and H7 subtype viruses become highly pathogenic avian influenza viruses and highly transmittable among chickens. These subtype viruses cause a high fatality rate.
A genetic reassortant virus can cause a pandemic in humans
Humans can rarely be infected with avian influenza viruses. The virus has not been transmitted between humans to date, so it has never caused a pandemic. Then, what caused the pandemics in the past: the Spanish flu in 1918; Asian flu in 1957; Hong Kong flu in 1968; and the 2009 pandemic flu?
Kida strongly suspected influenza was a zoonosis after reading academic articles by overseas researchers pointing to similarities between a viral protein found in ducks and horses, and that of the 1968 Hong Kong pandemic influenza virus strain. He examined feces of migratory birds and finally, in 1977, he isolated a virus (A/duck/Hokkaido/5/77) found in the intestine of a duck that had flown from Siberia to Hokkaido. This virus had HA identical to that of the 1968 Hong Kong influenza virus and NA undistinguishable from that of the 1957 Asian flu virus.
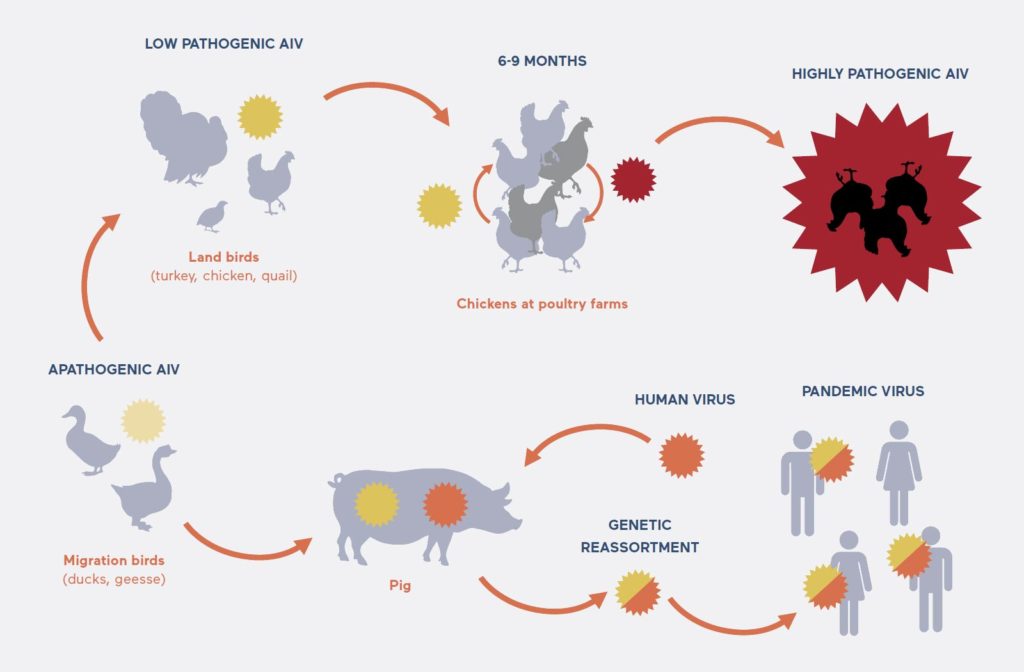
How avian and human pandemic virus strains emerge: An apathogenic avian influenza virus (AIV) carried by wild ducks is transmitted to poultry such as geese, quails and turkeys and becomes low pathogenic. After the virus is transmitted among chickens over six months, the virus becomes highly pathogenic (HPAIV). A human pandemic virus emerges after a pig contracts both avian and human influenza viruses, and produces hybrid viruses through genetic reassortment.
(Adobe Stock © marinavorona, © ssstocker)
“This duck taught me that the emergence of a new virus is not a result of antigenic variation, but genetic reassortment between different strains,” Kida said. Genetic reassortment is the process by which influenza viruses swap gene segments. Ensuing experiments showed that two of the eight viral genes originated in ducks and the six others were from the Asian flu virus.
“We proved pigs, which have receptors both to the avian and human viruses, serve as a mixing vessel,” Kida said. “When pigs are simultaneously infected with both influenza viruses, the genetic reassortment will take place, producing an ‘avian-human hybrid virus’ that can infect and transmit between humans. Among them, those with HA genes that originated in ducks are pandemic influenza viruses.”
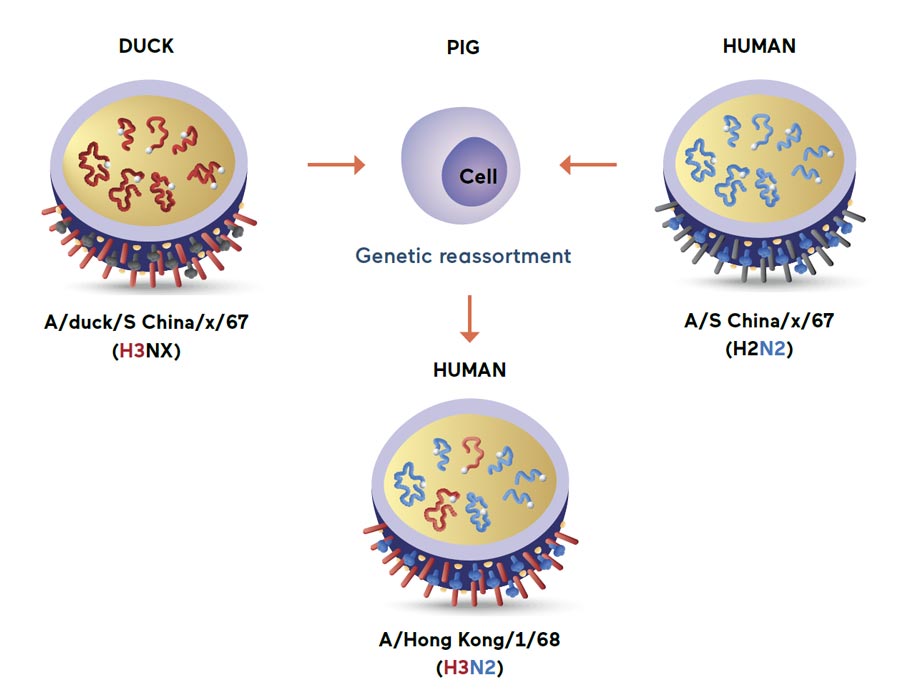
In this genetic reassortment, an avian virus, H3NX, carried by a duck, was concurrently with a human H2N2 influenza virus to generate the 1968 Hong Kong pandemic influenza virus.
(Adobe Stock © designua)
Pandemic flu virus highly transmittable, but not so pathogenic
At least the past four pandemic influenza viruses were proven to have come from pigs. During the 2009 pandemic, there were ungrounded fears that more than 500,000 people would die from the flu in Japan alone, which led to the enactment of special legislation to deal with pandemic influenza.
“I strongly opposed the government’s move because the virus that proliferates in pigs will not propagate as much in the human body,” Kida said. “But since humans do not have immunity against the pandemic virus, it is highly transmissible, albeit not so pathogenic. Transmissibility and pathogenicity are totally different things.”
The 1918 Spanish flu was allegedly so pathogenic that it caused the deaths of 40 million people, but later studies showed many of these deaths were caused by simultaneous infection with bacteria and poor living conditions, Kida said.
Kida laments the wide spread of what he considers to be a gross misunderstanding of pandemic influenza. “The media still incorrectly report that a new type of influenza emerges as a result of mutations acquired by avian influenza viruses.”
Kida said a flu virus strain is the collection of mutant viruses in the first place. While they transmit from human to human, some mutant viruses that are more proliferative than others will be dominant. A pandemic flu then becomes a seasonal one next year and beyond, becoming more pathogenic in humans.
Trying to dispel misunderstanding over influenza
Kida said understanding how people get sick from influenza is crucial. There is a widespread misperception that the virus itself causes sickness in the human body. “Sickness occurs as innate immune responses when the amount of a virus increases in the body,” Kida said. “When excessive responses occur, a person runs a fever or develops serious symptoms. In the most acute cases, blood vessels can be damaged by clots and vascular hyperpermeability, resulting in multiple organ failure, including encephalopathy in children.”
It is vital to control pandemic influenza based on countermeasures against seasonal influenza, Kida stressed. Accordingly, improving seasonal flu vaccines is urgently needed. Kida is determined to realize the introduction of whole virus particle vaccines to replace the weak immunogenic split vaccines that are currently used.
One of Kida’s many contributions is accumulating influenza virus data from duck feces to make a library of pandemic influenza vaccine strain candidates. The library stores more than 4,600 avian influenza virus strains of all 144 combinations of HA and NA subtypes as vaccine strain candidates. Their pathogenicity, antigenicity, genetic information and yield in chicken embryo have been analyzed, put in a database and made available on a website (https://virusdb.czc.hokudai.ac.jp/).
“I declined suggestions that the database be patented,” Kida said. “It is my responsibility to make the database available to whoever needs it, because I cannot achieve much by myself as a researcher.”
The library of pandemic influenza vaccine strain candidates: Black viruses were isolated from a waterfowl (81 combinations), while red ones were generated in the laboratory (63 combinations). More than 4,600 avian influenza virus strains of 144 combinations of HA and NA subtypes are stored in the library.
Click here to see the table of contents.