Cancer stem cells trigger macrophage aging
Cancer stem cells cause the aging of macrophages in mice with healthy immune systems, creating conditions for the formation of tumors.

Cancerous tumors consist of a mixture of cells, the most important of which are cancer stem cells. These cells are capable of establishing new cancerous tumors by evading the immune response. Research has focused on identifying biomarkers for cancer stem cells and developing therapies that target these cells. Unfortunately, candidate drugs developed from these efforts have so far not been very effective in clinical trials.
A research team led by Associate Professor Haruka Wada at Hokkaido University’s Institute for Genetic Medicine examined the mechanisms by which cancer stem cells evade immune response in mice models. They showed that cancer stem cells induce senescence in macrophages—the immune cells which are responsible for the first step of the destruction of cancer cells. Their findings were published in the Journal for ImmunoTherapy of Cancer.
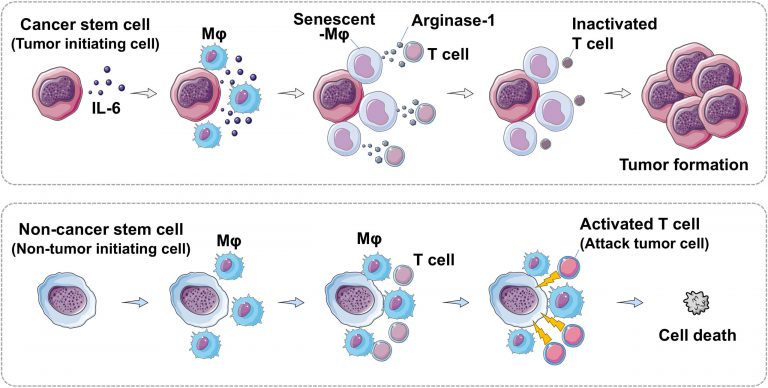
“One of the biggest questions in the development of cancer is how cancer develops in individuals with a healthy immune system,” explains Wada. “The majority of studies on cancer stem cells have been carried out in vitro or in immunodeficient mice models, which do not account for a fully functioning immune response. The lack of effectiveness of cancer stem cell-targeting drugs indicates that the immune response or lack thereof is more important than previously considered.”
The team used two cell lines of glioblastoma tumor, one of which was capable of inducing tumor formation (cancer stem cell) and the other of which was not. In mice models, the cancer stem cells suppressed the proliferation of macrophages; further investigation showed that macrophages cultured with cancer stem cells exhibit senescence or cellular aging. Macrophages were not the only immune cells affected; while the proliferation of T cells was unchanged, their antitumor activity was suppressed due to the immunosuppressive factors produced by senescent macrophages. The team identified interleukin 6 (IL-6) produced by cancer stem cells as the molecule responsible for triggering these effects.
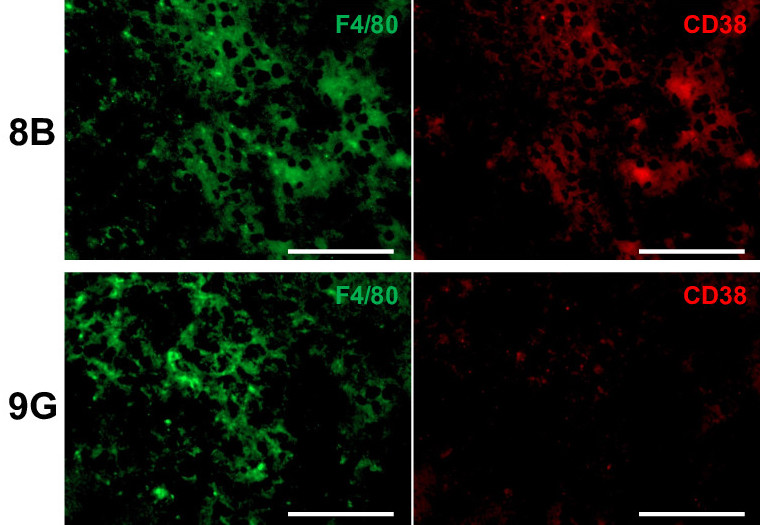
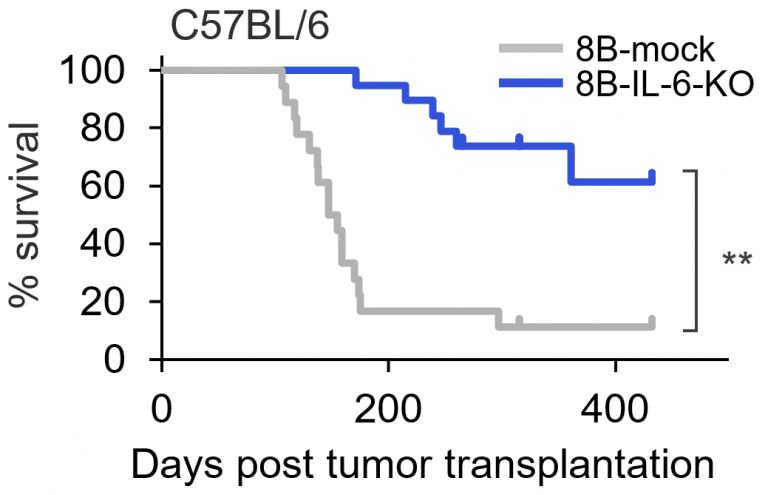
The team also demonstrated that supplementing the mice inoculated with cancer stem cells with a molecule called nicotinamide mononucleotide resulted in the proliferation of non-senescent macrophages and reduced the immunosuppressive factors produced by senescent macrophages, preventing tumor growth and leading to increased survival times in mice.
“Our results indicate that drugs targeting senescent macrophages could be a treatment for cancer—an unprecedented development,” concluded Wada. “We believe that these drugs could be part of a treatment that prevents the new onset of tumors, as well as a therapy that prevents recurrence after cancer treatment.” Future work will focus on two avenues: confirming that this discovery holds true for cancers other than glioblastomas, and confirming that the findings apply to cancers in humans.

Original Article:
Haruka Wada, et al. Tumor cell-induced macrophage senescence plays a pivotal role in tumor initiation followed by stable growth in immunocompetent condition. Journal for ImmunoTherapy of Cancer. November 14, 2023.
DOI: 10.1136/jitc-2023-006677
Funding:
This study was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (26640066, 16K18408, 26640099); the Japan Agency for Medical Research and Development (AMED; 19bm0404028h0002, 19ck0106262h0003); the Kato Memorial Bioscience Foundation; Suhara memorial foundation; The Akiyama life science foundation; Friends of Leukemia Research Fund; The Mitsubishi Foundation; and Joint Research Program of the Institute for Genetic Medicine, the project of junior scientist promotion, and the Photo-excitonix Project in Hokkaido University.
Contacts:
Associate Professor Haruka Wada
Institute for Genetic Medicine
Hokkaido University
Tel: +81-11-706-5532
Email: wada[at]igm.hokudai.ac.jp
Sohail Keegan Pinto (International Public Relations Specialist)
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Tel: +81-11-706-2186
Email: en-press[at]general.hokudai.ac.jp
