Scientists discover a single-electron bond in a carbon-based compound
Joint release by Hokkaido University and the University of Tokyo.
The discovery of a stable single-electron covalent bond between two carbon atoms validates a century-old theory.

Covalent bonds, in which two atoms are bound together by sharing a pair of electrons, form the scaffolding that underpins the majority of organic compounds. In 1931, the Nobel Laureate Linus Pauling suggested that covalent bonds made from just a single, unpaired electron could exist, but these single-electron bonds would likely be much weaker than a standard covalent bond involving a pair of electrons.
Since then, single-electron bonds have been observed, but never in carbon or hydrogen — the hunt for one-electron bonds shared between carbon atoms has stymied scientists.
Now, a team of researchers from Hokkaido University has isolated a compound in which a single electron is shared between two carbon atoms in a remarkably stable covalent bond, known as a sigma bond. Their findings are published in the journal Nature.
“Elucidating the nature of single-electron sigma-bonds between two carbon atoms is essential to gain a deeper understanding of chemical-bonding theories and would provide further insights into chemical reactions,” explains Professor Yusuke Ishigaki, of the Department of Chemistry at Hokkaido University, who co-authored the study.
The single-electron bond was formed by subjecting a derivative of hexaphenylethane, which contains an extremely stretched out paired-electron covalent bond between two carbon atoms, to an oxidation reaction in the presence of iodine. The reaction produced dark violet-colored crystals of an iodine salt.
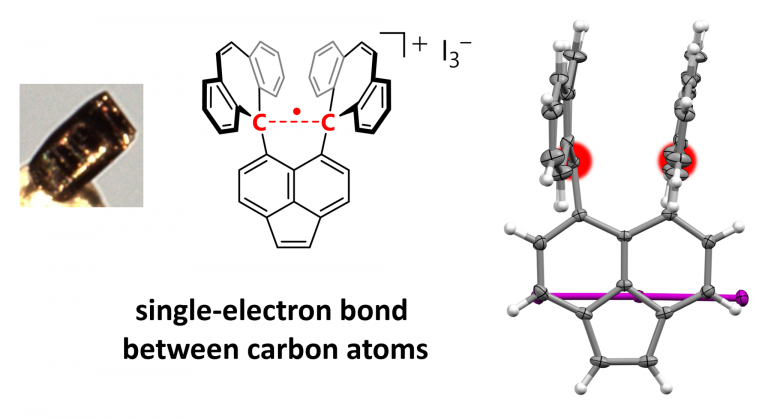
The team used X-ray diffraction analysis to study the crystals and found that the carbon atoms in them were extremely close together, suggesting the presence of single-electron covalent bonds between carbon atoms. They were then able to confirm this using a form of chemical analysis called Raman spectroscopy.
“These results thus constitute the first piece of experimental evidence for a carbon-carbon single-electron covalent bond, which can be expected to pave the way for further developments of the chemistry of this scarcely-explored type of bonding,” says Takuya Shimajiri, the lead author of the paper and now at the University of Tokyo.

Original Article:
Takuya Shimajiri, et al. Direct evidence for a carbon–carbon one-electron σ-bond. Nature. September 25, 2024.
DOI: 10.1038/s41586-024-07965-1
Funding:
This work was supported by the Masason Foundation; the Research Program “Five-star Alliance” within “NJRC Mater. & Dev.” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; a Toyota Riken Scholarship; Grants-in-Aid from MEXT (Japan) and the Japan Society for the Promotion of Science (JSPS; JP23K13726, JP23K20275, JP23K21107, JP23H04011); and the Japan Science and Technology Agency (JST) PRESTO (No. JPMJPR23Q1).
Contacts:
Assistant Professor Takuya Shimajiri
School of Science
The University of Tokyo
Tel: +81-3-5841-8061
Email: shimajiri[at]chem.s.u-tokyo.ac.jp
Associate Professor Yusuke Ishigaki
Faculty of Science
Hokkaido University
Tel: +81-11-706-2701
Email: yishigaki[at]sci.hokudai.ac.jp
Sohail Keegan Pinto (International Public Relations Specialist)
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Tel: +81-11-706-2186
Email: en-press[at]general.hokudai.ac.jp
Emese Berta
School of Science
The University of Tokyo
Email: media.s[at]gs.mail.u-tokyo.ac.jp
