Facile method for carboxylic acid activation using inexpensive commercial organic photocatalysts
Photoactivated ketones prove to be promising H-atom transfer photocatalysts for activating carboxylic acids and generating new C–C, C–S, and C–Cl bonds.
Carboxylic acids are ubiquitous in bioactive organic molecules and readily available chemical building blocks. Carboxylic acids can be converted into carboxy radicals that can initiate versatile carbon-carbon and carbon-heteroatom bonds formations, which is highly desirable for developing materials and pharmaceuticals. Currently, however, there are few applicable methods that use inexpensive catalysts. To this end, researchers from WPI-ICReDD and University of Shizuoka have developed a facile hydrogen atom transfer (HAT) method that selectively transforms carboxylic acids into carboxy radicals using xanthone, an inexpensive commercial organic ketone photocatalyst. This research was published in the Journal of the American Chemical Society.
HAT converts substrates into radical species by removing a hydrogen atom and ketones are highly accessible, inexpensive, and known for HAT photocatalysis. However, selective HAT for carboxylic acids is challenging because the O–H bond is stronger than adjacent C–H bonds. Nonetheless, using the artificial force induced reaction (AFIR) method, a computational technique developed at ICReDD, the authors identified xanthone as a promising ketone photocatalyst for selective O–H bond HAT.
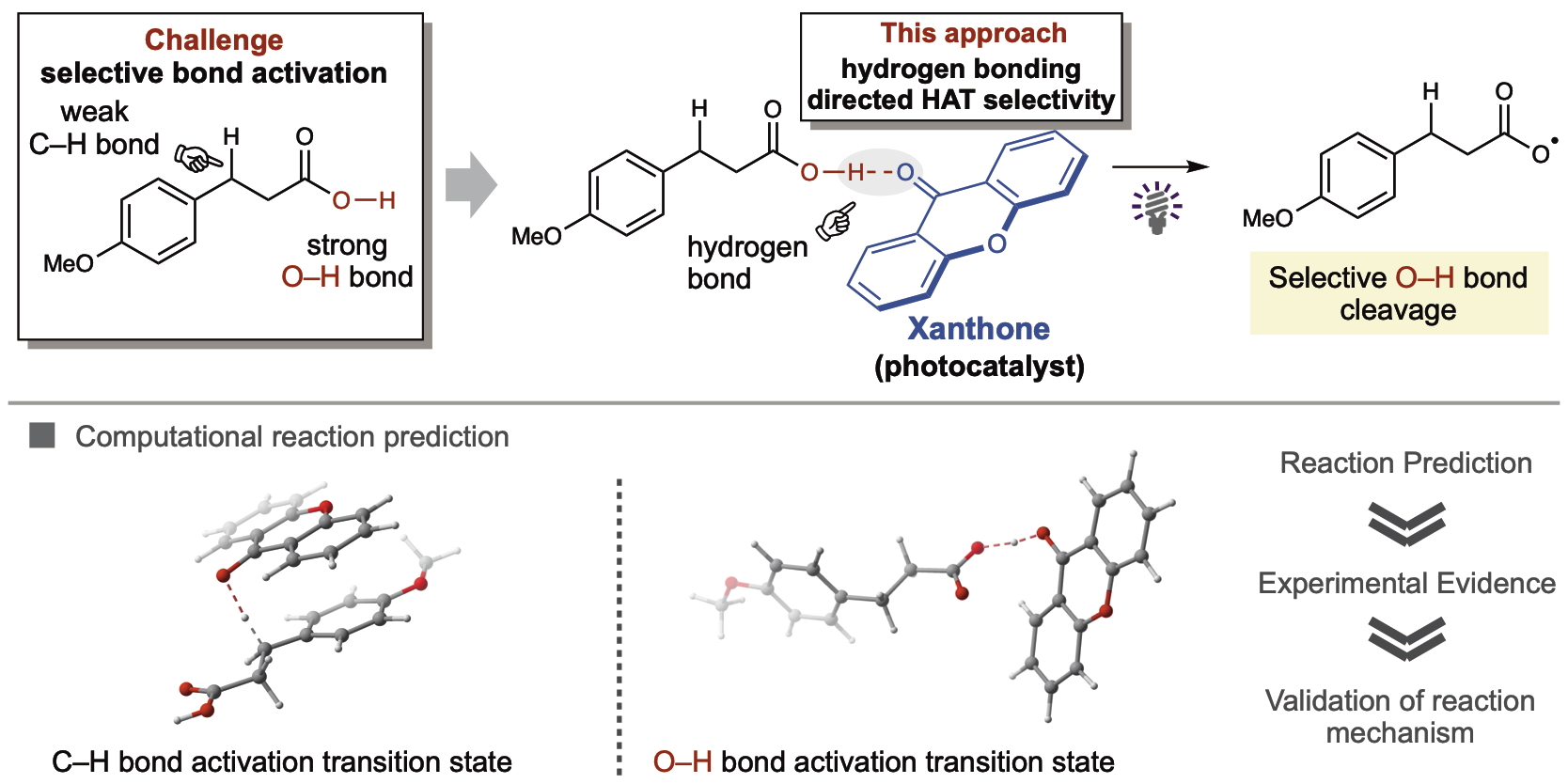
The computational calculations revealed that xanthone has similar energy barriers for activating either O–H or C–H bonds in carboxylic acid substrates. As such, hydrogen bonding would be capable of influencing product selectivity. Hydrogen bonding is an attraction that can occur between oxygen and hydrogen atoms (depicted above) but does not occur with carbon atoms. Not to be confused with a covalent bond.
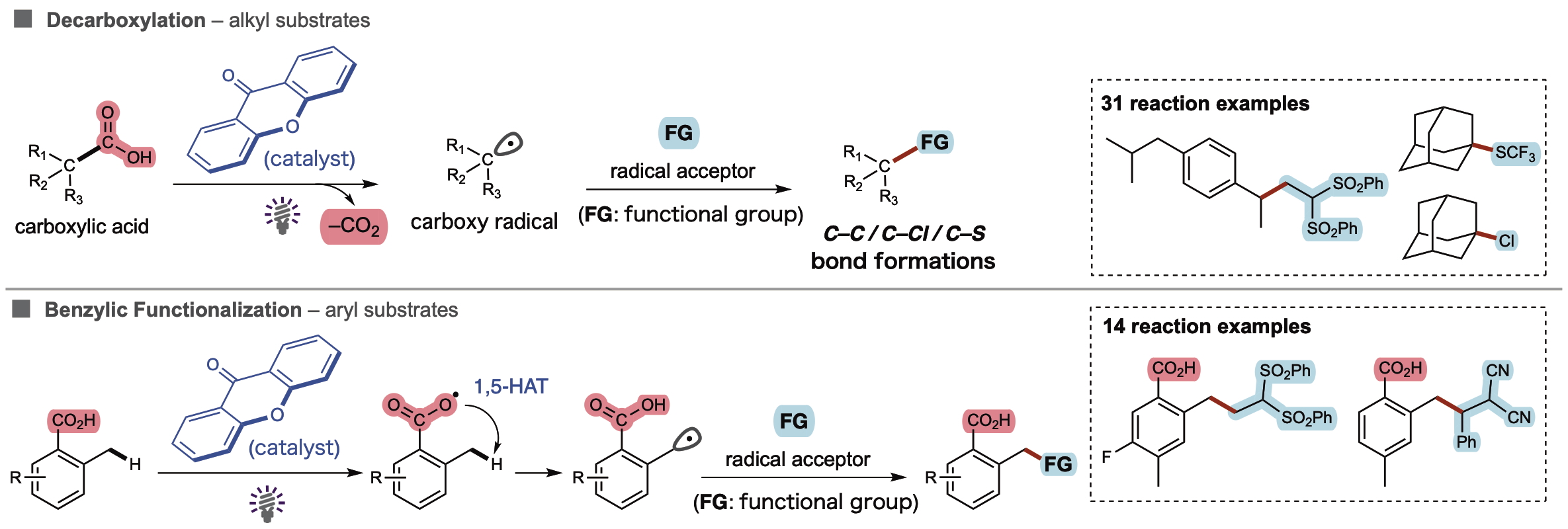
The AFIR predictions and the mechanistic significance of hydrogen bonding were experimentally validated with >10:1 ratios for selective O–H bond cleavage. The reaction proceeded through decarboxylation or benzylic functionalization depending on the substrate. The xanthone demonstrated impressive substrate compatibility, versatile bond formations (C–C, C–Cl, and C–S bonds), and established broad applicability with >40 reaction examples.
“Collaborating with the team in Shizuoka to explore a new activation mode of carboxylic acids through computational approaches was an excellent experience. It’s especially meaningful to me that the AFIR method has demonstrated its utility as a predictive tool beyond ICReDD. I hope this computational strategy will continue to drive new advances in reaction development,” said Associate Professor Hiroki Hayashi from WPI-ICReDD at Hokkaido University.
“I’m deeply honored to have collaborated with Associate Professor Hiroki Hayashi on this work. By integrating both experimental and computational approaches, we successfully revealed a novel photocatalysis of ketones. This project made me truly appreciate the power of the AFIR method in predicting reaction pathways. I hope our findings will open new avenues for controlling highly reactive radical species,” said Assistant Professor Kenji Yamashita from University of Shizuoka.
This new methodology is inexpensive and generates minimal reaction waste making it highly accessible for developing pharmaceuticals and materials. Moreover, the photocatalytic mechanism of this method is promising for broader radical generation applications besides carboxy radicals.
Original article:
Kenji Yamashita*, Hayate Sano, Yuki Goto, Hiroki Hayashi*, and Yoshitaka Hamashima*. Direct Generation of Carboxyl Radicals from Carboxylic Acids Catalyzed by Photoactivated Ketones. Journal of the American Chemical Society. July 3, 2025.
DOI: 10.1021/jacs.5c04571
Funding:
This work was supported by grants from University of Shizuoka, Grants-in-Aid for Scientific Research (B) (22H02745), Grant-in-Aid for Challenging Research (Exploratory) (21K18965) , a grant from the Naito Foundation, JSPS-WPI, JST-ERATO (JPMJER1903) , and Transformative Research Areas (A) Green Catalysis Science for Renovating Transformation of Carbon-Based Resources (Green Catalysis Science) (24H01830) .
Contacts:
Professor Hiroki Hayashi
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Tel: +81-11-706-9654
Email: hhayashi[at]icredd.hokudai.ac.jp
Samuel Jacob (Public Relations and Outreach)
Institute for Chemical Reaction Design and Discovery
Hokkaido University
Tel: +81-11-706-9646
E-mail: public_relations[at]icredd.hokudai.ac.jp
Naoki Namba
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Tel: +81-11-706-2185
Email: en-press[at]general.hokudai.ac.jp
