Double network hydrogel polymers with rapid self-strengthening abilities
New double network hydrogel technology features automated self-strengthening that rapidly activates upon deformation of its polymer network.
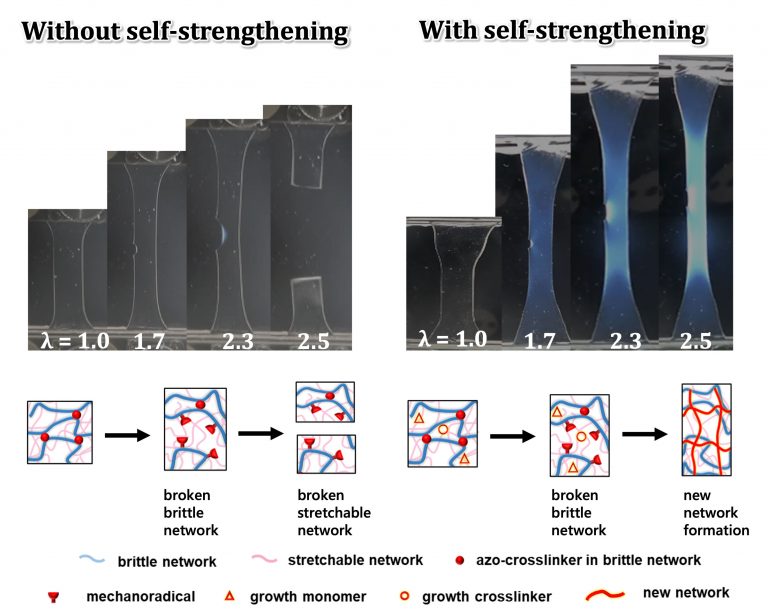
Hydrogels are soft materials consisting of polymer networks and water. They are permeable to substances smaller than their network mesh size and have applications in biomaterials, contact lenses, soft robots, and more. At the molecular level, the cleavage of chemical bonds causes a material to become mechanically weaker and can lead to its destruction. Mechanochemically cleaved bonds usually result in the formation of mechano-radicals. In the presence of monomers, these mechano-radicals can trigger the formation of new polymer networks, self-strengthening the material.
Professor Jian Ping Gong’s research team at the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, previously achieved such self-strengthening in double network (DN) hydrogels consisting of a hard, brittle primary polymer network and a flexible, stretchable secondary polymer network. However, these self-strengthening mechanisms were too sluggish to provide reinforcement during typical deformation rates. Addressing this, Professor Gong’s research team hasve recently developed a rapid self-strengthening hydrogel technology using weak chemical bond cleavage for activation.
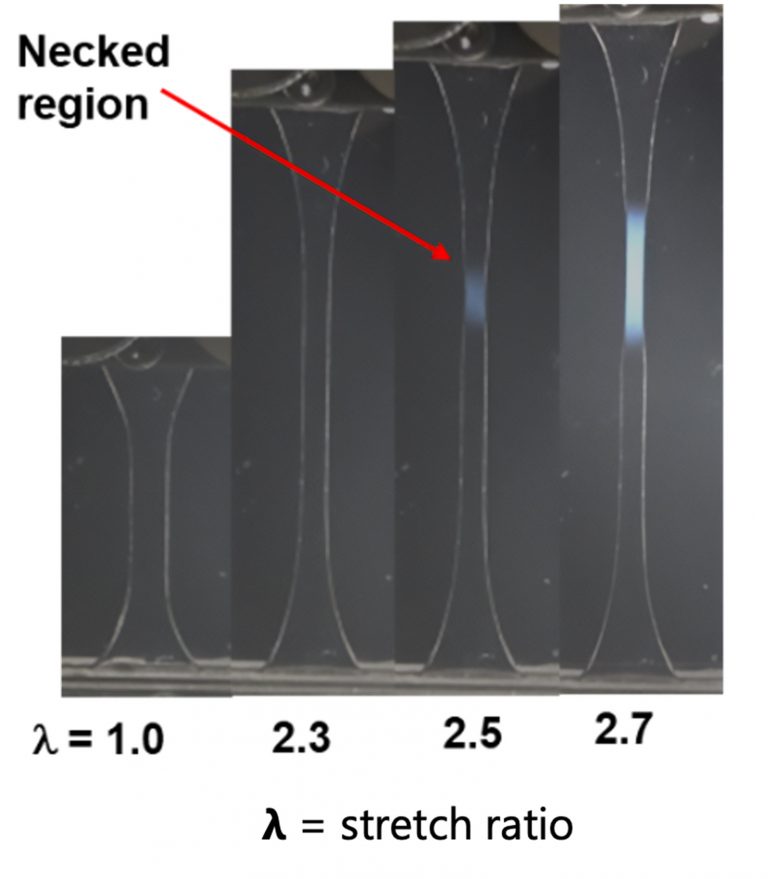
For this, they modified a DN hydrogel by embedding molecular components containing notably weak azo bonds (–N=N–) into the primary polymer network. Subsequently, the hydrogel’s permeable properties were utilized, allowing it to soak up a large concentration of monomers and crosslinkers (i.e., the molecular precursors for polymer growth).
Deformation of the material by mechanical loading (e.g., stretching) induces a mechanochemical reaction that selectively breaks the weak azo bonds embedded in the primary network generating reactive mechano-radicals. These radicals rapidly react with the absorbed monomers and crosslinkers, forming a new, stronger primary network. Notably, the rate of new network formation occurs rapidly, 100 times faster than the unmodified DN hydrogel, enhancing the material’s strength and preventing further destruction. In collaboration with Professor Michael Rubinstein’s theoretical group (WPI-ICReDD and Duke University), the authors determined that this rapid strengthening features significant rate dependence dictated by the interplay between deformation rate and the kinetics of new network formation.
“Self-strengthening materials mark a shift from the passive durability of force to active adaptation. By rationally utilizing mechanochemistry, especially by controlling its reaction kinetics, we can tailor the reinforcement to meet specific needs, not only in hydrogels but also in rubbers, elastomers, and beyond,” said the study’s first author, Zhi Jian Wang.
Original Article:
Zhi Jian Wang, et al. Rapid self-strengthening in double network hydrogels triggered by bond scission. Nature Materials. February 26, 2025.
DOI: 10.1038/s41563-025-02137-6
Funding:
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP22H04968, JP22K21342, JP24H00848); the Japan Science and Technology Agency (JST) FOREST (JPMJFR221X) and JST PRESTO (JPMJPR2098); and the US NSF Center for the Chemistry of Molecularly Optimized Networks (MONET) (CHE-2116298).
Contacts:
Professor Jian Ping Gong
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Tel: +81-11-706-9011
Email: gong[at]sci.hokudai.ac.jp
Samuel Jacob (Public Relations and Outreach)
Institute for Chemical Reaction Design and Discovery
Hokkaido University
Tel: +81-11-706-9646
E-mail: public_relations[at]icredd.hokudai.ac.jp
Sohail Keegan Pinto (International Public Relations Specialist)
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Tel: +81-11-706-2186
Email: en-press[at]general.hokudai.ac.jp
