Estrogen-negative cancers respond to anti-estrogenic therapies
Anti-estrogenic therapies can suppress the growth of cancer that does not express estrogen receptors; when combined with immune checkpoint inhibitor therapies, they halt tumor progression in mice models.
Estrogen, a group of female hormones, is known to be involved in cancer progression, especially breast cancer. About 75 % of breast cancers are estrogen-sensitive: they express the hormone receptor estrogen receptor α (ERα), and estrogen promotes tumor growth. Surprisingly, estrogen has been observed to promote tumor growth in ERα-negative cancers, such as triple-negative breast cancer (TNBC), for reasons that are not fully understood.
A team of researchers from the Institute for Genetic Medicine (IGM) at Hokkaido University has uncovered how estrogen affects the tumor microenvironment and promotes tumor growth in ERα-negative cancers. Their findings were published in the British Journal of Cancer.
“Generally speaking, estrogen directly affects cancer cells to promote cell survival and proliferation, and this has been considered to hold true only for estrogen-sensitive cancers,” explains Nabeel Kajihara, lead author of the paper. “Estrogen is also documented to play other roles in the tumor microenvironment, effectively suppressing the immune response and protecting tumors.”
The researchers analyzed patient data from The Cancer Genome Atlas (TCGA) and conducted experiments in cell cultures and mice models to understand what was happening.
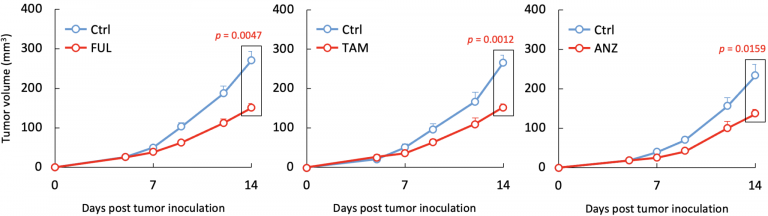
From the TCGA data, they observed that, in TNBC, estrogen suppresses the induction of cytotoxic T cells, which typically recognize and destroy cancer cells. They confirmed this observation in mice TNBC and colon cancer models, which do not have estrogen sensitivity; they then went one step further and studied the effects of anti-estrogenic therapy on these cancers in mice models. Therapy with fulvestrant, the most effective estrogen signal blocker currently approved for clinical use, suppressed the growth of tumor cells. Tumor growth was also suppressed by two other approved anti-estrogenic drugs, tamoxifen and anastrozole, confirming that estrogen signal-blocking was responsible.
Estrogen signal blockers modulate immune response to increase production of cytotoxic T cells, specifically by preventing the activity of estrogen. The team also showed that therapy combining estrogen signal blockers with a class of drugs called immune checkpoint inhibitors (ICIs) drastically suppresses tumor progression in mice models. Most notably, the combination of fulvestrant (anti-estrogenic) and anti-CTLA-4 (ICI) resulted in the complete suppression of TNBC tumor progression.
“We have demonstrated that anti-estrogenic therapies can potentially maximize therapeutic efficacy of cancer immunotherapy regardless of tumor cells’ ERα expression,” concludes Professor Seino. “However, this is only the first step; future clinical research is required to develop cancer therapies that utilize this approach.”

Original Article:
Nabeel Kajihara, Yunqi Ge, Ken-ichiro Seino. Blocking of oestrogen signals improves anti-tumour effect regardless of oestrogen receptor alpha expression in cancer cells. British Journal of Cancer. August 3, 2023.
DOI: 10.1038/s41416-023-02381-0
Funding:
This work was partly supported by Japan Science and Technology Agency (JST) SPRING (JPMJSP2119) and Japan Society for the Promotion of Science (JSPS) KAKENHI (22J21076).
Contacts:
Dr. Nabeel Kajihara
Division of Immunobiology
Graduate School of Medicine
Hokkaido University
Email: nabeel[at]igm.hokudai.ac.jp
Professor Ken-ichiro Seino
Division of Immunobiology
Institute for Genetic Medicine
Hokkaido University
Email: seino[at]igm.hokudai.ac.jp
Sohail Keegan Pinto (International Public Relations Specialist)
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Tel: +81-11-706-2186
Email: en-press[at]general.hokudai.ac.jp
Related Press Releases:
Root of Triple Negative Breast Cancer immunosuppression and chemoresistance revealed
Key Molecule Responsible for Poor Prognosis of Breast Cancer Identified
