Discovery of a mechanism of autonomous migration of epithelial cell sheets
Research Press Release | January 21, 2016
| Press Release | ||
|---|---|---|
| Key Points |
・Discovery of a mechanism by which epithelial cells can migrate while maintaining their attachment to adjacent cells. ・This leads to an understanding of the mechanisms by which the complex shapes of multicellular organisms are formed (morphogenesis). |
|
|
Background |
As multicellular organisms, we develop from a single fertilized egg, and we take on shape through many repeated cell divisions (morphogenesis). During this process our bodies are covered by an epithelial cell sheet that separates the inside of the body from the outside. This cell sheet must not break during morphogenesis because otherwise the inside of the body would mix with the outside. The cell sheet can autonomously bend, expand, and migrate during the process of morphogenesis (collective migration of epithelial cells). Deformation and migration of the cell sheet are basic to morphogenesis, so the understanding of these mechanisms is tied to the understanding of the mechanisms of morphogenesis. In prior research the autonomous bending and expanding of the cell sheet have been elucidated, but the mechanism that makes it possible for cells to autonomously migrate while maintaining attachments to adjacent cells had not been elucidated. We approached this problem from the viewpoint of mechanics. |
|
|
Research Methods |
Starting with a symmetry argument, we looked for conditions such as which properties of a cell make it possible to migrate in a uniform direction. Based on such considerations, we clarified that if there was chirality (such as when the sides of the cell experience a strong contractile force in a tilted direction) relative to the main axis of the cell body (for example, the axis joining the head and tail), then the cell would be able to migrate in one direction. We described the cell sheet by a mathematical model known as the vertex model, and incorporated the chirality of the cell. Our research is the first to introduce chirality into cells. |
|
|
Research Results |
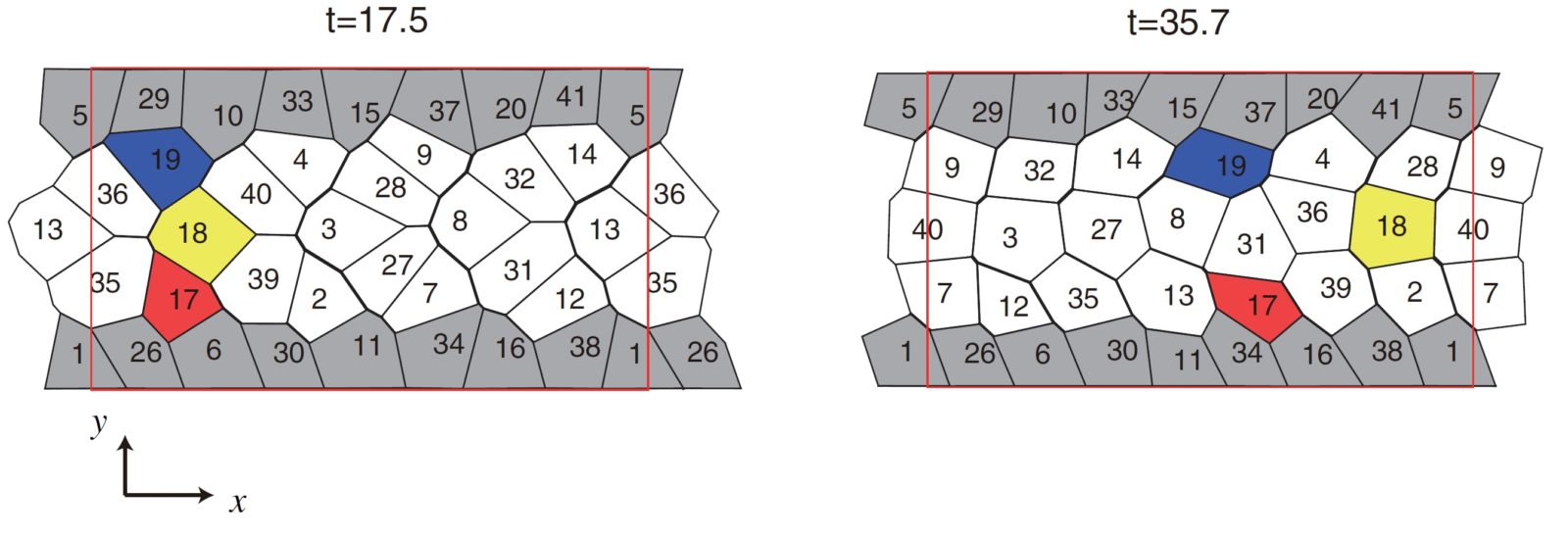
We showed that if cells have chirality, then the cells can continue to change its position while maintaining attachments to other cells. We also showed that when we changed the characteristics of the cells (for example, adhesive strength) in a direction that was perpendicular to the direction of migration, the entire cell sheet displayed the ability to migrate collectively and unidirectionally (Figure 1). |
|
|
Anticipated Outcomes |
The hypotheses of this mathematical model have been verified in the phenomenon of rotation of the genitalia in Drosophila pupae (Sato, K. et al., Nature Communications 6, 10074 (2015)). It is possible that similar mechanisms are also used in the formation of other organs in other species. Previous research into the phenomenon of morphogenesis, for which the method of migration was not understood, might become clear now if examined from the viewpoint of the chirality of cells. |
|
| Inquiries |
Physical Ethology Laboratory, Research Institute for Electronic Science, Hokkaido University Assoc. Prof. Katsuhiko SATO TEL:+81-11-706-9443 FAX:+81-11-706-9439 katsuhiko_sato[at]es.hokudai.ac.jp |
|
|
Japanese Link |
上皮細胞シートが自発的に移動する仕組みを発見 (01.04.2016) | |
| Publications | Cell Chirality Induces Collective Cell Migration in Epithelial Sheets, Physical Review Letters, (10.27.2015) | |
