Integrin α9 on lymphatic endothelial cell regulates lymphocyte egress
Research Press Release | February 26, 2014
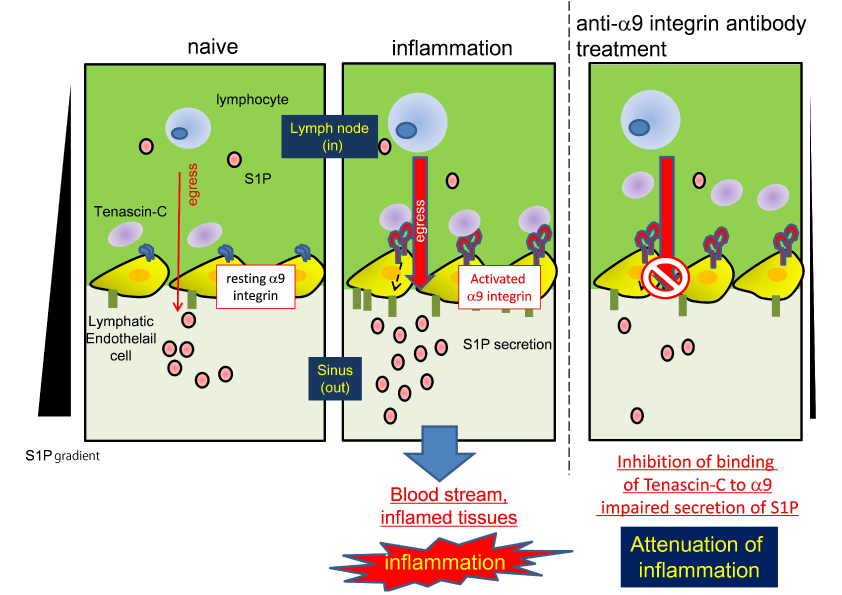
During inflammation (middle panel), the α9 integrin on lymphatic endothelial cells is activated, resulting in the enhancement of interaction of tenascin-C. This interaction leads to sphingosine-1 phosphate (S1P) and subsequent lymphocyte egress from the inflamed lymph nodes. In the lymph nodes of anti-α9 integrin antibody-treated mice with induced inflammation (right panel), this interaction was inhibited. As a result, S1P level in the sinus was low and lymphocyte egress was impaired. In a naïve condition, the α9 integrin on lymphatic endothelial cell is inactivated. Thus, in the naïve condition (left panel), lymphocyte egress is independent on the α9 integrin.
| Press Release | ||
|---|---|---|
| Key Points |
・We revealed the mechanism of α9 integrin-dependent lymphocyte egress from inflamed lymph nodes. ・Integrin α9 is a possible target for treatment of immune-mediated diseases, such as Multiple Sclerosis. |
|
| Overview |
In autoimmune diseases including Multiple Sclerosis, autoreactive cells are activated in the peripheral lymphoid organ. These cells exit the lymphoid organ, and migrate to a target organ (e.g.; the central nervous system in Multiple Sclerosis). Although it has been demonstrated that medullary and cortical sinuses-lining lymphatic endothelial cells, are the exit site of lymphocytes from lymph nodes, and express the α9 integrin, their role on lymphatic endothelial cells remains unclear. We found that tenascin-C, one of the binding molecules to the α9 integrin was co-localized with lymphatic endothelial cells, which suggests the possibility of specific interaction of it with tenascin-C on lymphatic endothelial cells. To analyze the biological function of the α9 integrin, our laboratory generated anti-murine α9 integrin antibodies (anti-α9). We found that CD4 T cell egress from lymph nodes was inhibited in anti-α9-treated mice with induced inflammation. This data indicates lymphocyte egress from inflamed lymph nodes is dependent on the α9 integrin. In addition, we isolated lymphatic endothelial cells from mice embryos and found that stimulation of lymphatic endothelial cells with tenascin-C induced secretion of sphingosine-1 phosphate (S1P), which is a critical regulator lipid of lymphocyte egress. Finally, for clinical relevancy, we tested the therapeutic effect in the development of autoimmune disease and found that treatment of anti-α9 attenuated clinical symptoms of experimental autoimmune encephalomyelitis (EAE), the mouse model of human Multiple Sclerosis. These results suggest that the α9 integrin might be a target for treatment of immune-mediated diseases through regulation of lymphocyte egress. |
|
| Inquiries |
Koyu Ito, Specially Appointed Assistant Professor, Department of Matrix Medicine, Institute for Genetic Medicine, Hokkaido University TEL:+81-11-706-5120 FAX:+81-11-706-7542 E-mail: ito@igm.hokudai.ac.jp |
|
|
Japanese Link |
||
| Publications | Proceedings of the National Academy of Sciences of the United States of America (2014.2.10) | |
