Toward tunable molecular switches from organic compounds
Research Press Release | April 07, 2023
Joint press release by Hokkaido University and Kyushu University.
Newly synthesized organic molecules can be tuned to emit different colors depending on their molecular structures in crystal form.

Crystals of the newly synthesized compounds, anthraquinodimethane derivatives, which have different colors depending on their molecular structure (Yusuke Ishigaki).
Molecular switches are chemicals with molecular structures that can be shifted between two or more stable configurations in response to changes in their environment. They are of great interest in the development of molecular computers, molecular machines and drug delivery systems. Compounds with conformational isomers—identical molecular formulas but different molecular structures—can make very effective molecular switches.
Researchers at Hokkaido University and Kyushu University have developed a technique to synthesize potential molecular switches from anthraquinodimethanes (AQDs), a group of overcrowded organic molecules. The study, led by Associate Professor Yusuke Ishigaki at Hokkaido University and Associate Professor Toshikazu Ono at Kyushu University, was published in the journal Materials Chemistry Frontiers.
“AQDs are a type of overcrowded ethylene, molecules with carbon-carbon double bonds surrounded by large chemical groups,” explains Ono. “They have two common isomers, the folded and twisted forms. They are especially interesting as molecular switches, as their sterically hindered double bond can provide isomers absorbing and emitting different wavelengths of light.”
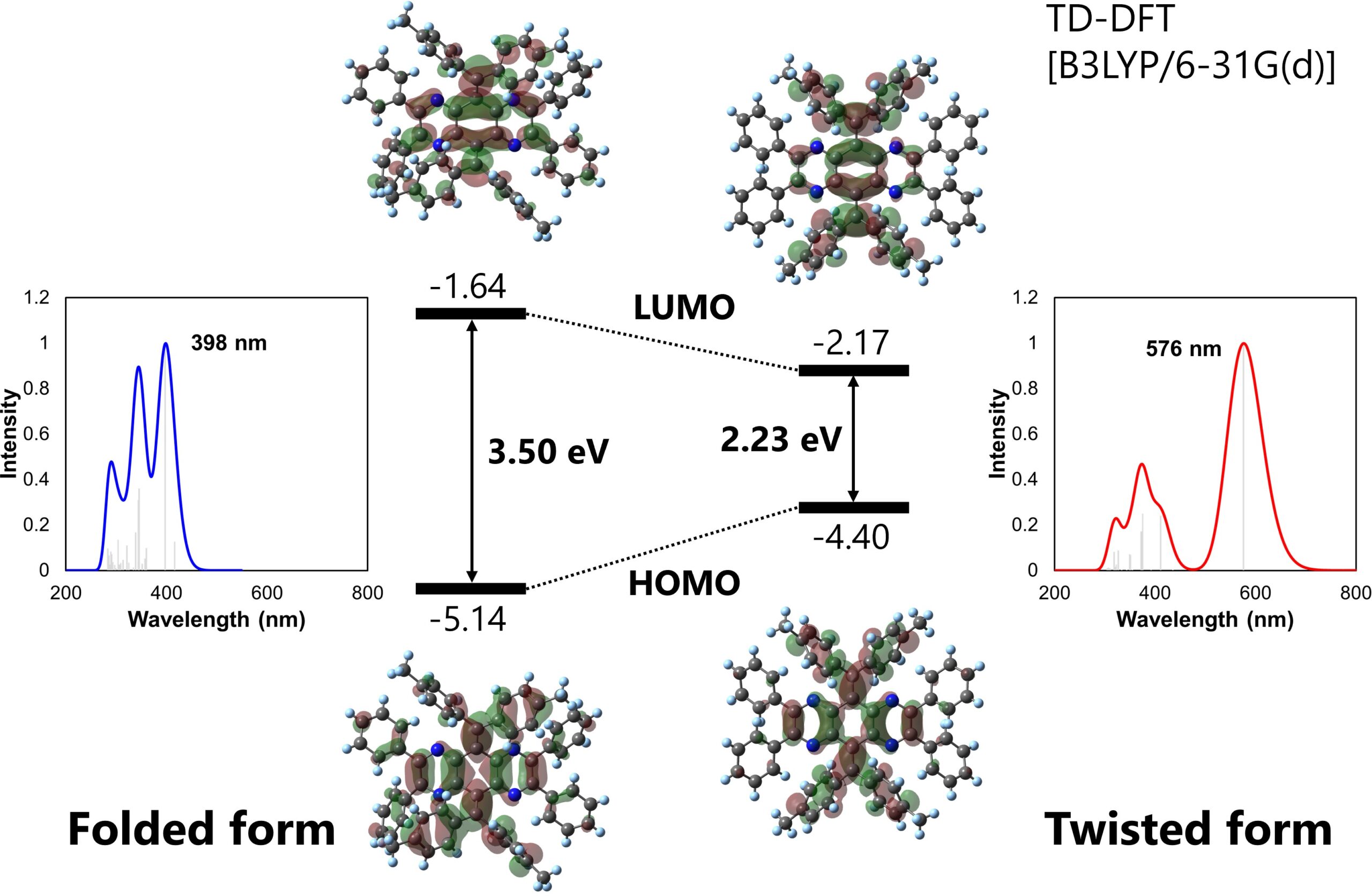
The folded and twisted isomers absorb different wavelengths of light (Kazuma Sugawara, et al. Materials Chemistry Frontiers. February 8, 2023).
AQDs generally adopt the most stable folded or twisted form, making it difficult to isolate pure samples of any other isomer to study its properties. The researchers surmounted this obstacle by designing flexible AQD derivatives that can more easily and stably form different isomers.
The synthesized derivatives were not only able to stably form twisted and folded isomers, but also other isomeric forms, when recrystallized in different solvents. The researchers performed detailed analysis of the derivatives to fully understand their properties.
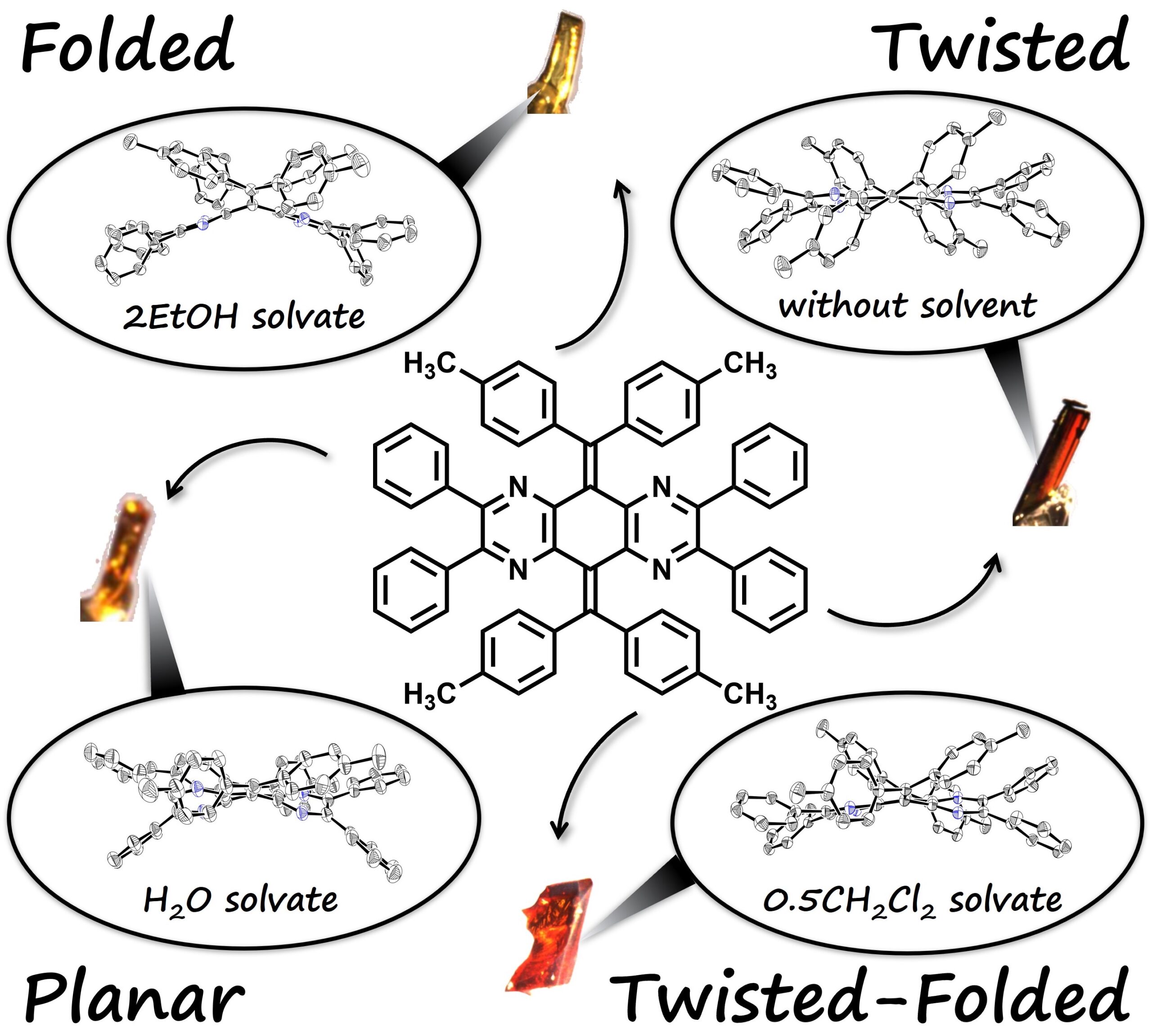
The methyl derivative of the new compound has four different isomers, with different crystal structures each (Kazuma Sugawara, et al. Materials Chemistry Frontiers. February 8, 2023).
In a crystalline state, each of these isomers absorbs and emits distinct frequencies of light, which is due to the differences in the distribution of electrons in the isomer molecules. Interestingly, the light absorption and emission changed when the crystals were ground into amorphous solid, and following treatment with appropriate solvents can produce original or other crystals with a variety of colors.
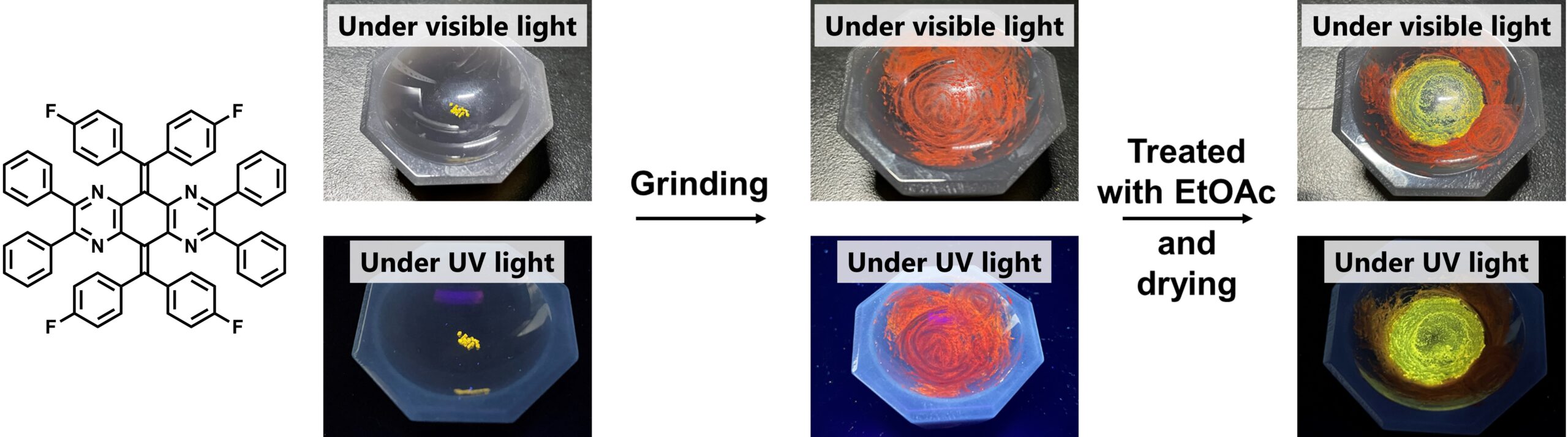
When ground into amorphous solid and treated with appropriate solvents the light absorption and emission changes (Kazuma Sugawara, et al. Materials Chemistry Frontiers. February 8, 2023).
“This work is the first report on the isolation of multiple isomeric forms of AQD,” Ishigaki concluded. “Their absorption and emission of different light frequencies, and more importantly, the ability to modulate the absorption and emission by external stimuli, make these compounds excellent candidates for the development of molecular switches.”
Original Article:
Kazuma Sugawara, et al. Exceptionally flexible quinodimethanes with multiple conformations: polymorph-dependent colour tone and emission of crystals. Materials Chemistry Frontiers. February 8, 2023.
DOI: 10.1039/D2QM01199A
Funding:
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI and Grant-in-Aid for Research Fellows (JP20H02719, JP20K21184, JP21H01912, JP21H05468, JP20J20972); Toyota Riken Scholar; and a 2020 DIC Award in Synthetic Organic Chemistry, Japan.
Contacts:
Researchers
Associate Professor Yusuke Ishigaki
Department of Chemistry
Faculty of Science
Hokkaido University
Tel: +81-11-706-2701
E-mail: yishigaki[at]sci.hokudai.ac.jp
Associate Professor Toshikazu Ono
Department of Applied Chemistry
Faculty of Engineering
Kyushu University
Tel: +81-92-802-2830
Email: tono[at]mail.cstm.kyushu-u.ac.jp
Institutions
Sohail Keegan Pinto (International Public Relations Specialist)
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Tel: +81-11-706-2186
Email: en-press[at]general.hokudai.ac.jp
Kyushu University Public Relations Initiative
Tel: +81-92-802-2443
email: sysintlkh[at]jimu.kyushu-u.ac.jp
Related Press Releases:
Carbon-carbon covalent bonds far more flexible than presumed
New record set for carbon-carbon single bond length


