Walking a tight line to study the properties of soft materials
Research Press Release | November 30, 2016
Tiny “walking” proteins could be used to investigate mechanical deformations in soft materials according to Hokkaido University researchers.
The researchers employed a concept that is a part of the transportation network in cells. “Walking” filament-shaped proteins, such as kinesin, carry cargo on one of their ends while two foot-shaped structures on the other end move one “foot” in front of the other along a network of associated protein microtubules in the cells.
The team aimed to test whether these walking proteins and their associated proteins could be used as a sensor for stretching and compressing a soft silicon-based material polydimethylsiloxane (PDMS) that is used, for example, in manufacturing contact lenses.
First, they deposited kinesin motor proteins on the surface of the PDMS so that one end of the kinesin proteins was attached to the PDMS, while the other end remained free. Next, the team deposited fluorescent-microtubules on the kinesin-layered surface. The layered PDMS was then placed in a special stretch chamber that was observed under the microscope.
When the team neither stretched nor compressed the PDMS, the microtubules moved randomly on the PDMS surface at a constant velocity. This movement effectively happens due to the kinesin proteins “walking in place” because they are attached to the surface of the PDMS. But as they move their “feet” along the free microtubules, the microtubules are forced to move around randomly on the material’s surface.
When the team stretched the PDMS, the microtubules moved faster and aligned themselves parallel to the stretching axis. The density of kinesin proteins on the surface also decreased as a result of the stretching. When the PDMS were compressed, however, the microtubules slowed down and aligned themselves perpendicular to the compression axis, while the density of kinesin proteins on the surface of the material increased.
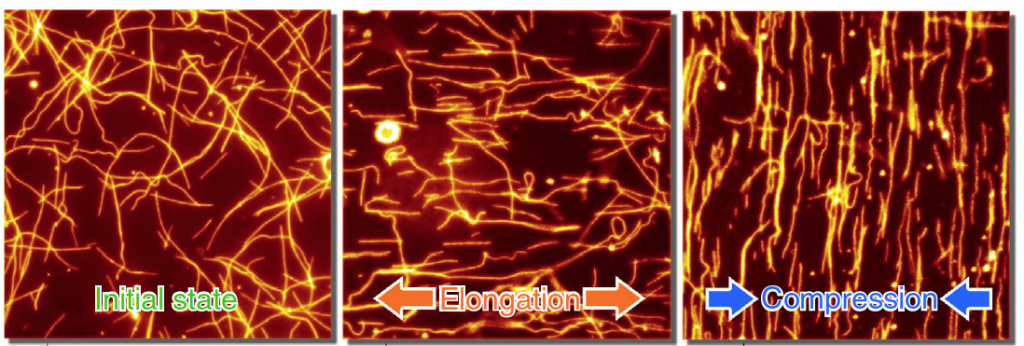
The team also tested the use of the microtubules as “probes” to detect the mechanical deformation of another soft material, polyurethane, a material commonly used in the manufacture of artificial skin and heart valves, and came up with similar results.
“Although further research is still required to prevent the denaturation of the proteins which occurs during the experiment, our present work should facilitate the elucidation of the surface science of soft materials in the future,” the researchers say in their study published in the journal Nature Communications. Akira Kakugo, the lead author of the paper, further explains: “since deforming soft materials provides environments that resemble living cells, our method could also help make clear the functions and mechanisms of motor proteins and microtubules interacting in the cells.”
Original paper:
Inoue D. et al., Sensing surface mechanical deformation using active probes driven by motor proteins. Nature Communications, October 3, 2016.
DOI: 10.1038/ncomms12557
Contacts:
Associate Professor Akira Kakugo
Department of Chemistry
Hokkaido University
Email: kakugo[at]sci.hokudai.ac.jp
Naoki Namba (Media Officer)
Global Relations Office
Institute for International Collaboration
Hokkaido University
Email: pr[at]oia.hokudai.ac.jp
Tel: +81-11-706-2185
